Question
Question: Given, \[N,N - \]dimethylaniline is treated with aqueous \(NaN{O_2}/HCl\), the product formed is: ...
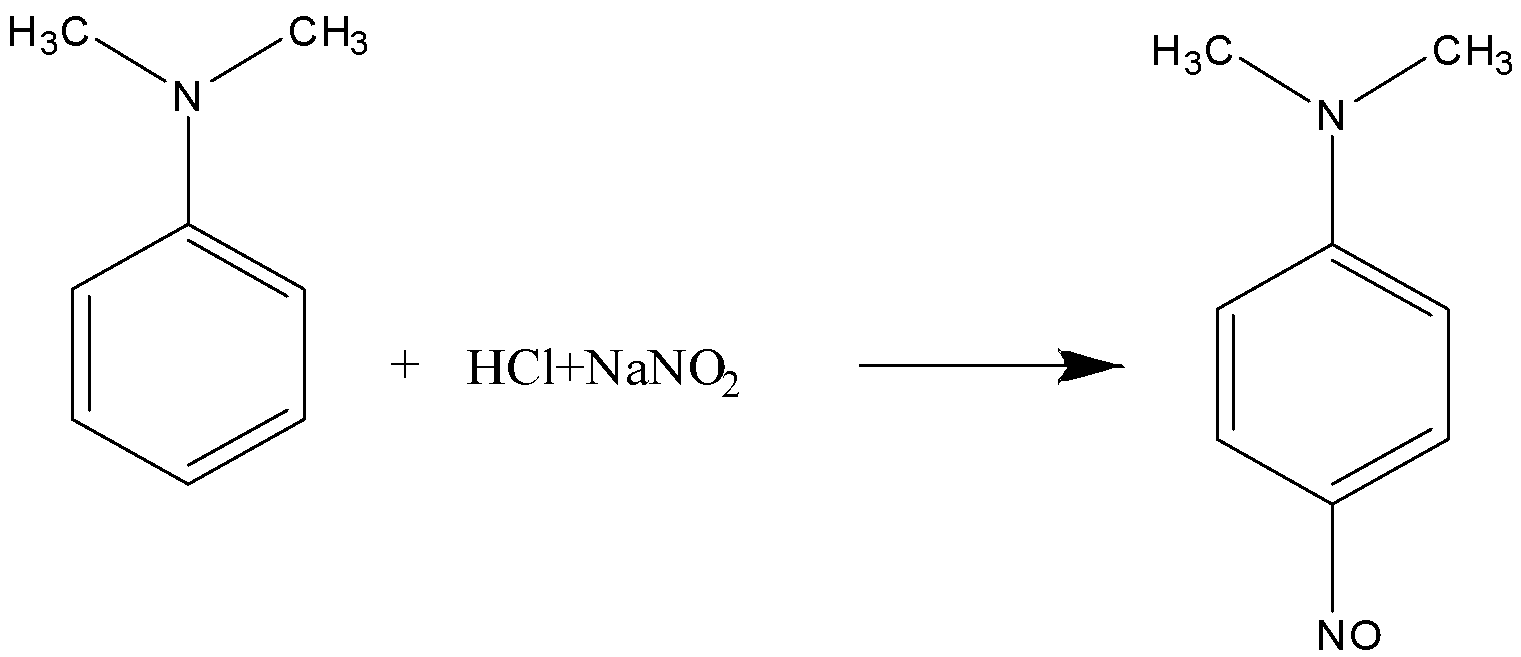
Given, N,N−dimethylaniline is treated with aqueous NaNO2/HCl, the product formed is:
A.p−(N,N−Dimethylamino) benzenediazonium chloride.
B.p−(N,N−Dimethylamino) phenol.
C.p−nitroso-N,N−dimethylaniline.
D.p−NitroN,N−dimethylaniline.
Solution
The reaction between N,N− dimethylaniline and NaNO2/HCl is a sandmeyer reaction. The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution.
Complete answer:
Sandmeyer reaction is a type of substitution reaction that is widely used in the production of aryl halides from aryl diazonium salts. Copper salts like chloride, bromide or iodide ions are used as catalysts in this reaction. Notably, the Sandmeyer reaction can be used to perform unique transformations on benzene.
Here, the N,N− dimethylaniline reacts with NaNO2/HCl and forms p−nitroso-N,N−dimethylaniline.
So, the correct answer is (C) p−nitroso-N,N−dimethylaniline
Additional information:
The most commonly employed Sandmeyer reactions are the chlorination, bromination, cyanation, and hydroxylation reactions using CuCl,CuBr,CuCN and CuO2, respectively. Diazonium salts also react with boronates, iodide, thiols, water, hypophosphorous acid and others, and fluorination can be carried out using tetrafluoroborate anions. However, since these processes do not require a metal catalyst, they are not usually referred to as Sandmeyer reactions.
Note:
The nitrous acid is typically prepared in situ from sodium nitrite and acid. In two protonation steps, one equivalent of water is lost to form the nitrosonium ion. The nitrosonium ion then acts as an electrophile in a reaction with an aromatic amine, such as aniline, to form a diazonium salt, proceeding through a nitrosamine intermediate.
