Question
Question: Given : <img src="https://cdn.pureessence.tech/canvas_112.png?top_left_x=930&top_left_y=894&width=3...
Given :
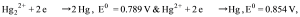
calculate the equilibrium constant for Hg22+→Hg + Hg2+ .
A
3.13 × 10-3
B
3.13 × 10-4
C
6.26 × 10-3
D
6.26 × 10-4
Answer
6.26 × 10-3
Explanation
Solution
+ 2e− →2Hg , 0.789 VoltHg → Hg2+ + 2e− , -0.854 VoltHg22+ →Hg + Hg2+ , -0.065 VoltΔ G = - 2× (- 0.065) × 96500 = -8.314 × 298 ln Keq. ;Keq.= 6.3 × 10-3
