Question
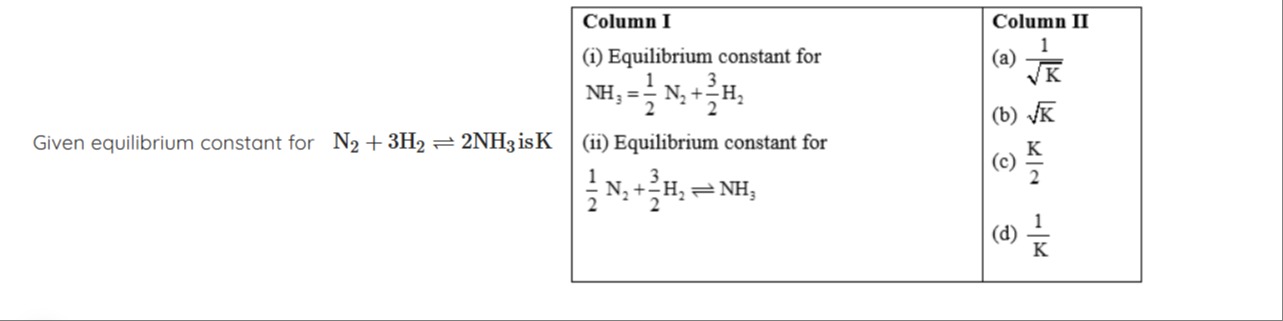
Question: Given equilibrium constant for $N_2 + 3H_2 \rightleftharpoons 2NH_3$ is K...
Given equilibrium constant for N2+3H2⇌2NH3 is K
Answer
(i) - (a), (ii) - (b)
Explanation
Solution
Let the given reaction be: N2+3H2⇌2NH3 The equilibrium constant is given as K. The expression for K is: K=[N2][H2]3[NH3]2
For the reaction in Column I (i): NH3⇌21N2+23H2 Let its equilibrium constant be Ki. The expression for Ki is: Ki=[NH3][N2]1/2[H2]3/2 We can see that Ki is the reciprocal of the square root of K: Ki=([N2][H2]3[NH3]2)−1/2=K−1/2=K1 Thus, (i) matches with (a).
For the reaction in Column I (ii): 21N2+23H2⇌NH3 Let its equilibrium constant be Kii. The expression for Kii is: Kii=[N2]1/2[H2]3/2[NH3] We can see that Kii is the square root of K: Kii=([N2][H2]3[NH3]2)1/2=K1/2=K Thus, (ii) matches with (b).
