Question
Question: Given, d- tartaric acid and l- tartaric acid are: A. geometrical isomers B. conformers C. enan...
Given, d- tartaric acid and l- tartaric acid are:
A. geometrical isomers
B. conformers
C. enantiomers
D. diastereomers
Solution
Tartaric acid is a 4 carbon organic compound that consists of two carboxylic acid groups. It is found in many fruits like tamarind. d and l configuration of any compound is its ability to rotate the plane polarized light towards right and left respectively.
Complete answer:
Tartaric acid has the formula of C4H6O6. Its open carbon chain structure can exist in two forms, one is with d- configuration form, while the other is l- configuration form. The difference in these forms is that they have an OH group on the right hand side in d- configuration, while on the left hand side in l- configuration.
The structures are, they are (R) l- configuration and (S)d- configuration respectively.
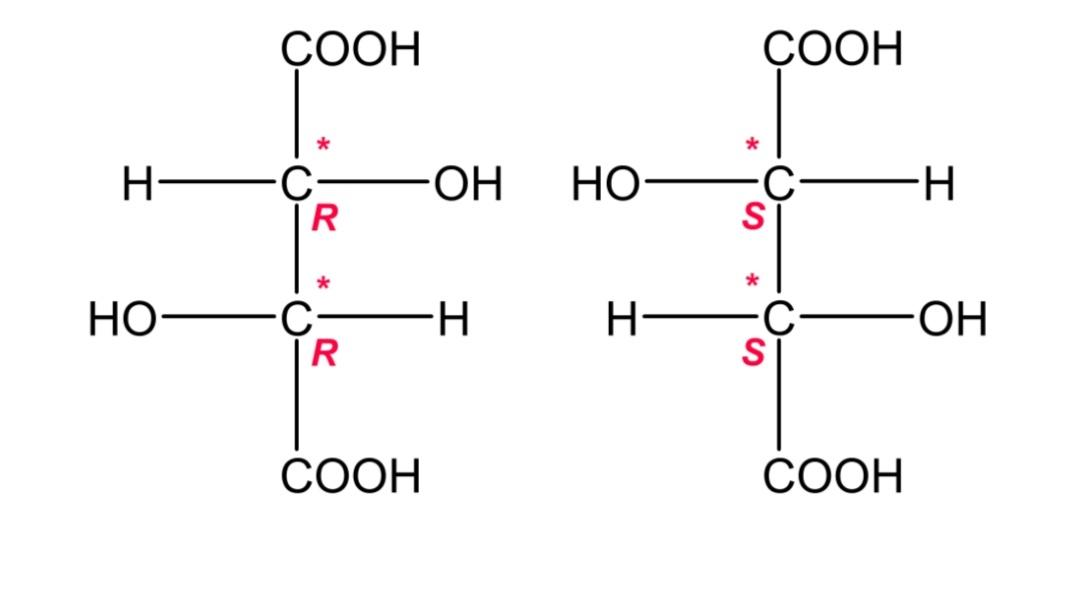
This d and l configuration is non- super imposable on each other, and is mirror images, so they are termed as enantiomers. Enantiomers are compounds that are mirror images but are non super impossible on each other. The structures that are mirror images are called enantiomorphs.
Hence, d- tartaric acid and l- tartaric acid are a pair of enantiomers.
Note:
The R and S are the carbon centers, the R center donates the rotation towards right, while the S center donates the rotation towards left. Geometrical isomers have the same molecular formula but different geometry, while conformers differ in their rotations, and diastereomers are non- super imposable, non mirror images of each other.
