Question
Question: Given, \(CH\equiv C-C{{H}_{3}}\xrightarrow[1%\text{ }HgS{{O}_{4}}]{40%\text{ }{{H}_{2}}S{{O}_{4}}}B\...
Given, CH\equiv C-C{{H}_{3}}\xrightarrow[1%\text{ }HgS{{O}_{4}}]{40%\text{ }{{H}_{2}}S{{O}_{4}}}B
Then, B is:
(a)- Acetone
(b)- Trichloroacetone
(c)- Acetaldehyde
(d)- Chloral
Solution
The given compound on the reactant side is propan-1-yne and it is a member of the alkyne group. When the alkynes react with 40% sulfuric acid and 1% of mercuric sulfate, then the hydration reaction occurs, i.e., there will be the addition of water molecules to the compound forming alcohol then, there will be tautomerism in the compound.
Complete answer:
The given compound on the reactant side is propan-1-yne and it is a member of the alkyne group because there are three carbon atoms in the chain and at the first carbon there is a triple bond.
When the alkynes react with 40% sulfuric acid and 1% of mercuric sulfate, then the hydration reaction occurs, i.e., there will be the addition of water molecules to the compound forming alcohol then, there will be tautomerism in the compound.
So, when the propyne reacts with 40% sulfuric acid and 1% of mercuric sulfate, then a hydrogen atom of the water gets added to the first carbon atom and the hydroxyl ion of water gets added to the second carbon atom forming alkene alcohol. Now, there will be tautomerism, i.e., the hydrogen atom will shift to the third position. So, the hydrogen atom of the hydroxyl group will move towards the CH2 group. The reaction is given below:
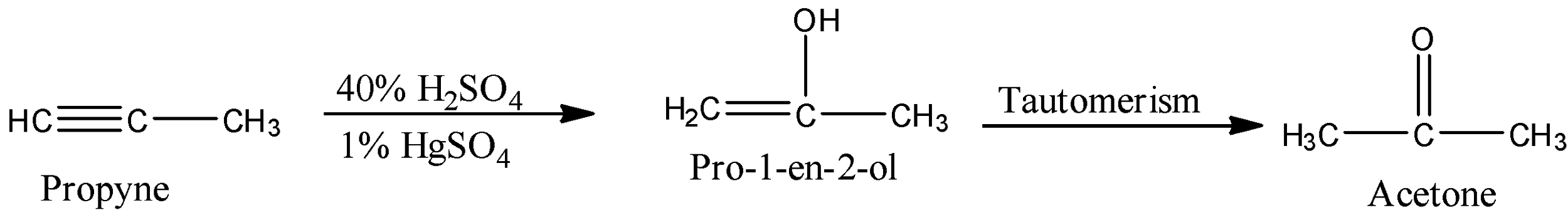
So, the product formed in the reaction is acetone.
Therefore, the correct answer is an option (a)- Acetone
Note:
The addition of water molecules to the alkyne takes place through Markovnikov’s rule, i.e., the negative part of the water molecule (hydroxyl ion) gets attached to the carbon atom having a lesser number of hydrogen atoms.
