Question
Question: Given, \(C{{H}_{3}}-CH=C{{H}_{2}}+HOH\xrightarrow{{{H}^{+}}}'x'\) Which of the following is ‘x’? ...
Given, CH3−CH=CH2+HOHH+′x′
Which of the following is ‘x’?
1)CH3CH2CH2OH
2)CH3CH2OH
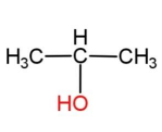
3) 
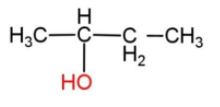
4)
Solution
The reaction of any alkene with water is called a hydration reaction. These types of reactions are in accordance with the markovnikov rule. These reactions take place in acidic or either alkaline mediums.
Complete answer:
We have been given a reaction where prop-1-ene reacts with a water molecule in the presence of an acid. This is a type of hydration reaction of alkenes. The reaction is also termed as addition of water to alkenes. These reactions take place according to the markovnikov rule, which states that the negative part of the addendum goes to that carbon of double bond having less number of hydrogen atoms.
So, the negative part from the water molecule which is the hydroxide ion, OH− moves to the second carbon of propene, as it has less number of hydrogen atoms, to form the product in option 3. So, the reaction will be as follows:
CH3−CH=CH2+HOHH+CH3−(CH)OH−CH3
The product formed is propan-2-ol.
Hence, option 3 is correct. Thus x is propan-2-ol.
Note:
The addition of water in alkenes follows the markovnikov rule, as water molecule is similar to that of halogen acid and consists of H-O bond similar to that of H-X bond in halogen acids. The reaction in the acidic medium occurs as the water forms hydronium ion, H3O+. Then the reaction happens in 3 steps, first step is the protonation of alkene, then the attachment of the water molecule to the unstable carbocation, and then the deprotonation from water molecule to form an alcohol.
