Question
Question: Given below is the part of a graph. a)Which gas law is related to this graph? b)Convert the valu...
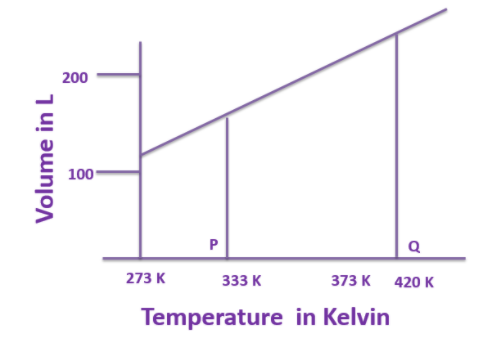
Given below is the part of a graph.
a)Which gas law is related to this graph?
b)Convert the values of temperature at ‘P’ and ‘Q’ given in Kelvin scale (K) to degree Celsius scale (O0C becomes double?
Solution
The gases which follow the ideal gas equation, PV=nRT are known as the ideal gases they follow the ideal gas equation at all the conditions of temperature and pressure. The gases which do not follow the ideal gas equation at all ranges of temperature and pressure are known as Real gases.
Complete step by step answer:
Certain laws are given by the different Scientists in order to determine the nature of the gases and the changes they undergo on the change in any one of the physical parameters such as temperature and pressure.
a)The graph given here resembles the graph of Charle's law. Since it is the graph between the Volume and temperature keeping the pressure constant. The Charles law was given in 1780 by Jacques Charles and it states that: “The volume of the gas (ideal gas) is directly proportional to the absolute temperature of the gas, keeping the pressure constant.” The mathematical statement of the law :
V∞T
Where V= Volume and T is the Temperature.
b)The temperatures given at point P and Q are 333 K and 420 K , in order to convert the temperature from Kelvin to the Celsius scale the following relationship can be used: Temperature(0C)=Temperature(K)−273
Thus the temperatures can be converted as:
1.At point P: The temperature is 333K
Temperature(0C)=Temperature(K)−273 =333−273 =600C
Similarly the temperature at point Q can also be calculated:
2.At point Q: The temperature is 420K
Temperature {(^0}C) = Temperature(K) - 273 \\\
= 420 - 273 \\\
= {47^0}C \\\
Note:
The Charle’s law has wide applications, some of its applications in the day to day life include in the cold weathers the helium balloons shrink and the capacity of the lungs decreases during winters and thus in turn the jogging becomes difficult in the winters.
