Question
Question: Given below are two reactions of water with sodium and carbon dioxide, what is the nature of water i...
Given below are two reactions of water with sodium and carbon dioxide, what is the nature of water in these reactions?
(i)2Na+2H2O→2NaOH+H2
(ii)6CO2+12H2O→C6H12O6+6H2O+6O2
A. In (ii) water acts as an oxidizing agent and in (i) it acts as a reducing agent
B. In (i) water acts as an oxidizing agent and in (ii) it acts as a reducing agent
C. In both (i) and (ii) hydrogen acts as a reducing agent.
D. In both (i) and (ii) hydrogen acts as an oxidizing agent
Solution
Oxidation is the process that occurs in an atom, ion, or molecule when there is a loss of electrons.
Oxidation number in an atom of a molecule is defined as the residual charge which an atom has or appears to have when all other atoms in a molecule or ion are removed as ions. The oxidation number in an atom may be negative, positive, or zero.
A substance which supplies oxygen or removes hydrogen is called an oxidizing agent.
A substance which supplies hydrogen and removes oxygen is called a reducing agent.
According to the electron concept, a chemical reaction in which there is a transfer of electrons from one substance (atom, ion, or molecule) to another substance is called an oxidation-reduction reaction or redox reaction.
Complete step by step answer:
The first reaction is written below:
2Na+2H2O→2NaOH+H2
In the above reaction the oxidation state of hydrogen of the water molecule in the reactant part is +1 and in the product part, the oxidation state of hydrogen is 0. Therefore, we can say that reduction is taking place in the above reaction. The one which gets reduced is called an oxidizing agent and hence hydrogen of the water molecule gets reduced in this case so water will act as an oxidizing agent.
The second reaction is written below:
6CO2+12H2O→C6H12O6+6H2O+6O2
In the above reaction, the oxidation state of oxygen of the water molecule in the reactant part is −2 and in the product part, the oxidation state of oxygen is 0. Therefore, we can say that oxidation is taking place in the above reaction. The one which gets oxidized is called a reducing agent and hence oxygen of the water molecule gets oxidized in this case so water will act as a reducing agent.
After discussing it we can conclude that in (i) water acts as an oxidizing agent and in (ii) it acts as a reducing agent.
So, the correct answer is Option B.
Note: Water was considered to be an element until 1781. However, Cavendish in 1781, showed that it can be obtained by burning hydrogen. Therefore, it is not an element. Lavoisier (1781) confirmed the compound nature of water. Davy (1800) showed that water is composed of hydrogen and oxygen in the ratio 2:1 by volume. Dumas (1842) established that water contains hydrogen and oxygen in the ratio 1:8 by weight.
The structure of the water molecule is
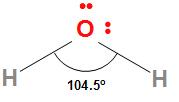
The H−O−H bond angle in water is 104.5∘ and the O−H bond length is 95.7 pm. Because of the high electronegativity difference between oxygen and hydrogen, the O−H bond in water is polar. The oxygen due to high electronegativity has a partial negative charge and the hydrogen atoms due to their lowest electronegativity have partial positive charges. The dipole moment of water is 1.84 D which confirms its polar nature.
