Question
Question: Given, $A^{2+}(aq) + 2e^{-} \rightleftharpoons A(s) \quad E^{\circ} = -0.96 V$ $ACl_2(s) + 2e^{-} \...
Given, A2+(aq)+2e−⇌A(s)E∘=−0.96V
ACl2(s)+2e−⇌A(s)+2Cl−(aq)E∘=−1.1V
Find log10Ksp1 (report to the nearest integer), here Ksp is the solubility product of ACl2(s) (F2.303RT=0.0591)
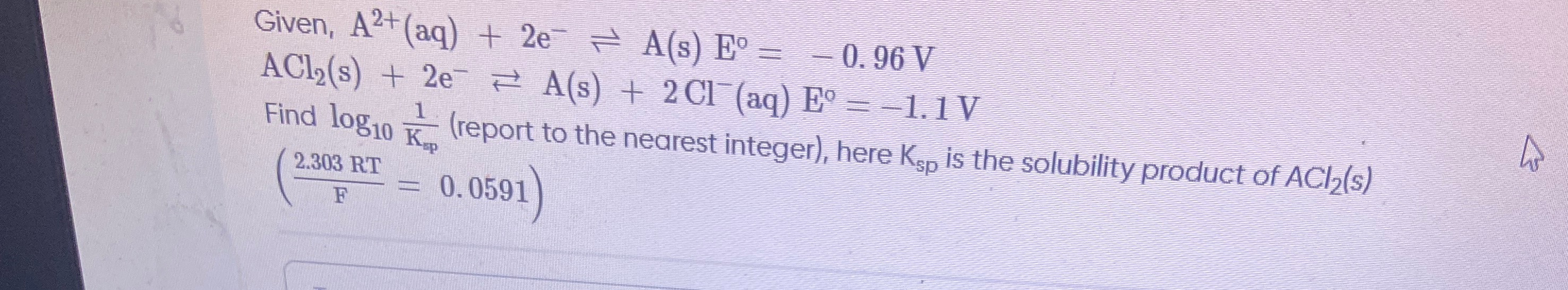
5
Solution
To find the solubility product constant (Ksp) for ACl2(s), we need to relate the given standard electrode potentials to the dissolution reaction of ACl2.
The dissolution reaction for ACl2(s) is: ACl2(s)⇌A2+(aq)+2Cl−(aq)
We are given the following standard electrode potentials:
- A2+(aq)+2e−⇌A(s)E1∘=−0.96V
- ACl2(s)+2e−⇌A(s)+2Cl−(aq)E2∘=−1.1V
To obtain the dissolution reaction, we can subtract the first half-reaction from the second half-reaction: (ACl2(s)+2e−⇌A(s)+2Cl−(aq)) −(A2+(aq)+2e−⇌A(s))
ACl2(s)−A2+(aq)⇌2Cl−(aq)
Rearranging this gives the dissolution reaction: ACl2(s)⇌A2+(aq)+2Cl−(aq)
The standard cell potential (Ecell∘) for this reaction is the difference between the standard electrode potentials: Ecell∘=E2∘−E1∘ Ecell∘=(−1.1V)−(−0.96V) Ecell∘=−1.1V+0.96V Ecell∘=−0.14V
This Ecell∘ is related to the equilibrium constant (Ksp in this case) by the Nernst equation at standard conditions: Ecell∘=nF2.303RTlog10Ksp
We are given (F2.303RT=0.0591). In the dissolution reaction ACl2(s)⇌A2+(aq)+2Cl−(aq), the number of electrons transferred (n) is 2 (as derived from the half-reactions).
Substitute the values into the equation: −0.14V=20.0591Vlog10Ksp −0.14=0.02955log10Ksp
Now, solve for log10Ksp: log10Ksp=0.02955−0.14 log10Ksp≈−4.7377
The question asks for log10Ksp1. log10Ksp1=−log10Ksp log10Ksp1=−(−4.7377) log10Ksp1=4.7377
Rounding to the nearest integer, 4.7377 is approximately 5.
