Question
Question: Given,\[2 - methyl - pentan - 1 - ol\] is A) \[1^\circ \] alcohol B) \[2^\circ \] alcohol C) ...
Given,2−methyl−pentan−1−ol is
A) 1∘ alcohol
B) 2∘ alcohol
C) 3∘ alcohol
D) None of these
Solution
Similar to water, an alcohol can be pictured as having an sp3 hybridized tetrahedral oxygen atom with nonbonding pairs of electrons occupying two of the four sp3 hybrid orbitals. Alcohol classification is an application of the neutral bonding patterns for organic compounds. Oxygen can only form two bonds.
Complete answer:
The structure of 2-methyl-pentan-1-ol is-
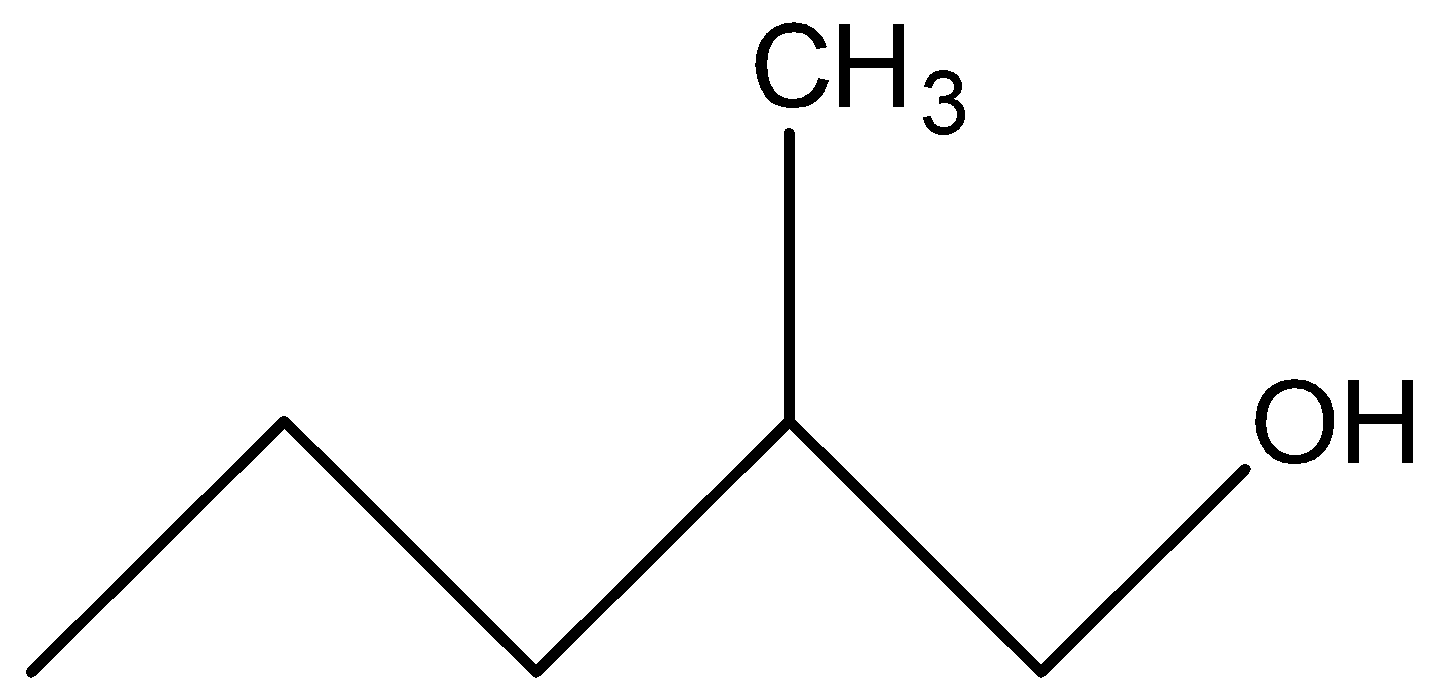
Classification of alcohols-
Alcohol classification is an application of the neutral bonding patterns for organic compounds. Oxygen can only form two bonds. The alcohol functional group requires that one of these bonds form with hydrogen to create the hydroxyl group and the other bond needs to be with carbon to create an alcohol. All of the oxygen atoms of all the alcohols look the same, so a different distinction is needed.
Methyl alcohol = no (0) other carbon atoms (CH3OH).
Primary alcohols ( 1∘) = 1 other carbon atoms
Secondary alcohols ( 2∘) = 2 other carbon atom
Tertiary alcohols ( 3∘) = 3 other carbon atoms
So from the above discussion we can say that 2−methyl−pentan−1−ol is a primary alcohol.
Alcohols are referred to as allylic or benzylic if the hydroxyl group is bonded to an allylic carbon atom (adjacent to a C=C double bond) or a benzylic carbon atom (next to a benzene ring), respectively.
Note:
The common name of an alcohol combines the name of the alkyl group with the word alcohol. If the alkyl group is complex, the common name becomes awkward and the IUPAC name should be used. Common names often incorporate obsolete terms in the naming of the alkyl group; for example, amyl is frequently used instead of pentyl for a five-carbon chain.
