Question
Question: Given,1-Butene and cyclobutane shows:- (A) Position isomerism (B) Ring-Chain isomerism (C) Fun...
Given,1-Butene and cyclobutane shows:-
(A) Position isomerism
(B) Ring-Chain isomerism
(C) Functional isomerism
(D) Metamerism
Solution
As we know that isomerism is the phenomenon where more than one compound has the same chemical formula but completely different chemical structures. Also chemical compounds which have identical chemical formulas but differ in physical and chemical properties due to different arrangement of atoms in the molecule are called isomers. So here we have to tell the isomerism between 1-Butene and cyclobutane.
Complete answer:
Let us discuss about isomerism followed by structural isomerism and its types as follows:-
-Isomerism: It is the phenomenon where more than one compound has the same chemical formula but completely different chemical structures. It is generally of two types i.e., structural isomerism and stereoisomerism.
-Structural isomerism: It is also known as constitutional isomerism and it is the phenomenon where compounds have the same molecular formula but atoms or groups are linked in different manner. It is of following types:-
Chain isomerism: It is also referred to as skeletal isomerism and components (atoms or groups) of these isomers display differently branched structures. These mainly differ in the branching of carbon atoms in the chain.
Position isomerism: In this isomerism, the position of the functional groups or atoms is linked to different carbon.
Functional isomerism: It refers to the compounds which have the same chemical formula but different functional groups attached to them.
Metamerism: It is the type of isomerism which arises due to the presence of different alkyl chains on each side of the functional group.
Ring-Chain isomerism: It is the type of isomerism where one compound has open chain structure and other has a ring structure.
-Now let check the isomerism between 1-Butene and cyclobutane:-
Structure of 1-Butene is given below:-
CH2=CH−CH2−CH3
Structure of cyclobutane is given below:-
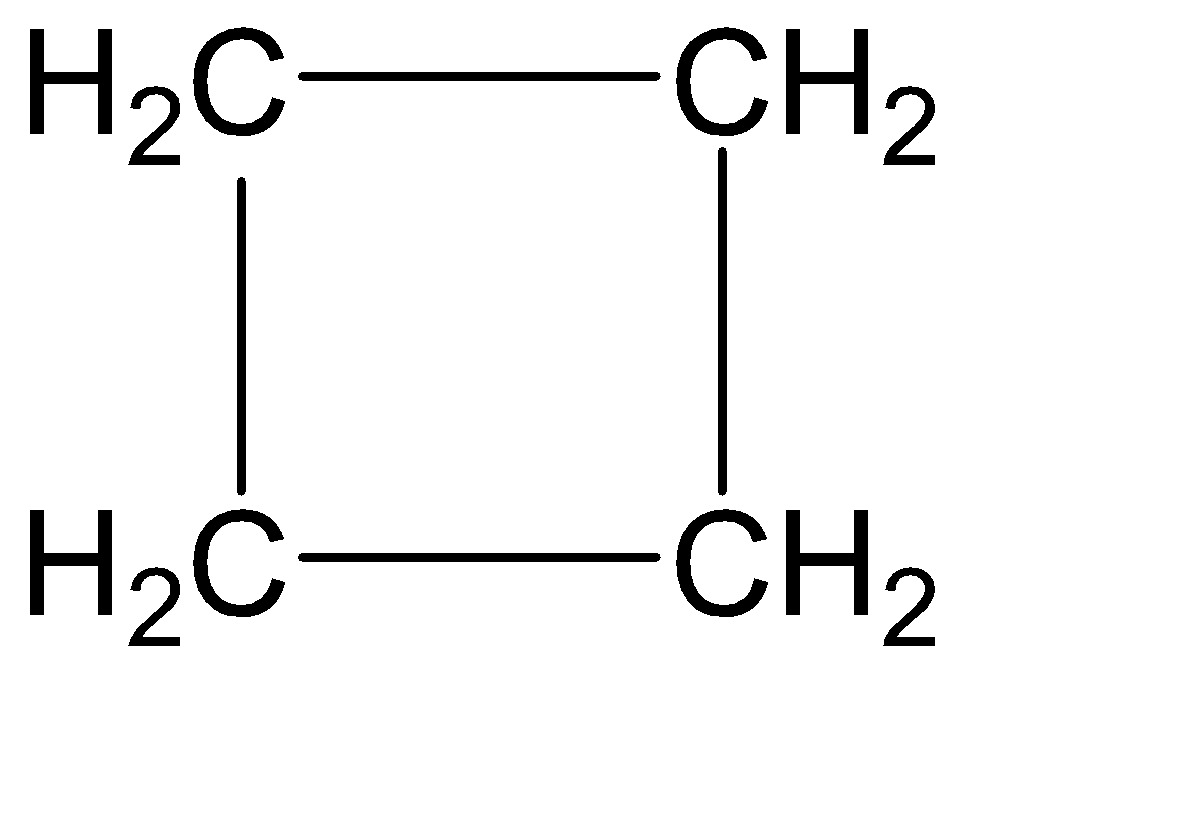
As we can see that the chemical formula of both the compound is same that is C4H8 but 1-Butene has open chain structure whereas cyclobutane has a ring structure which means that they show Ring-Chain isomerism.
Therefore 1-Butene and cyclobutane shows: (B) Ring-Chain isomerism.
Note:
-Remember that generally the isomers that show ring-chain isomerism can have different numbers of pi bonds.
-Also learn and understand about different types of isomerism shown by the compounds in organic chemistry to solve these types of questions.
