Question
Question: Give two examples for oxidation-reduction reaction....
Give two examples for oxidation-reduction reaction.
Solution
The name ‘oxidation-reduction reaction’ indicates that in this reaction both oxidation and reduction reactions take place. Another name of the oxidation-reduction reaction is redox reaction.
Complete step by step answer:
Let’s first understand the oxidation number. It is the total count of electrons that an atom loses or gains to result in a chemical bond.
Now we discuss oxidation and reduction reactions. Oxidation is the reaction in which oxidation number of atoms increases by losing electrons.
Now we define the reduction reaction. It is the reaction in which oxidation number of atoms decreases by gaining electrons.
Now, come to the question. We are asked to give two examples of oxidation-reduction reactions. We know that oxidation reduction reactions are those reactions in which both the oxidation and the reduction reaction take place.
Example 1)
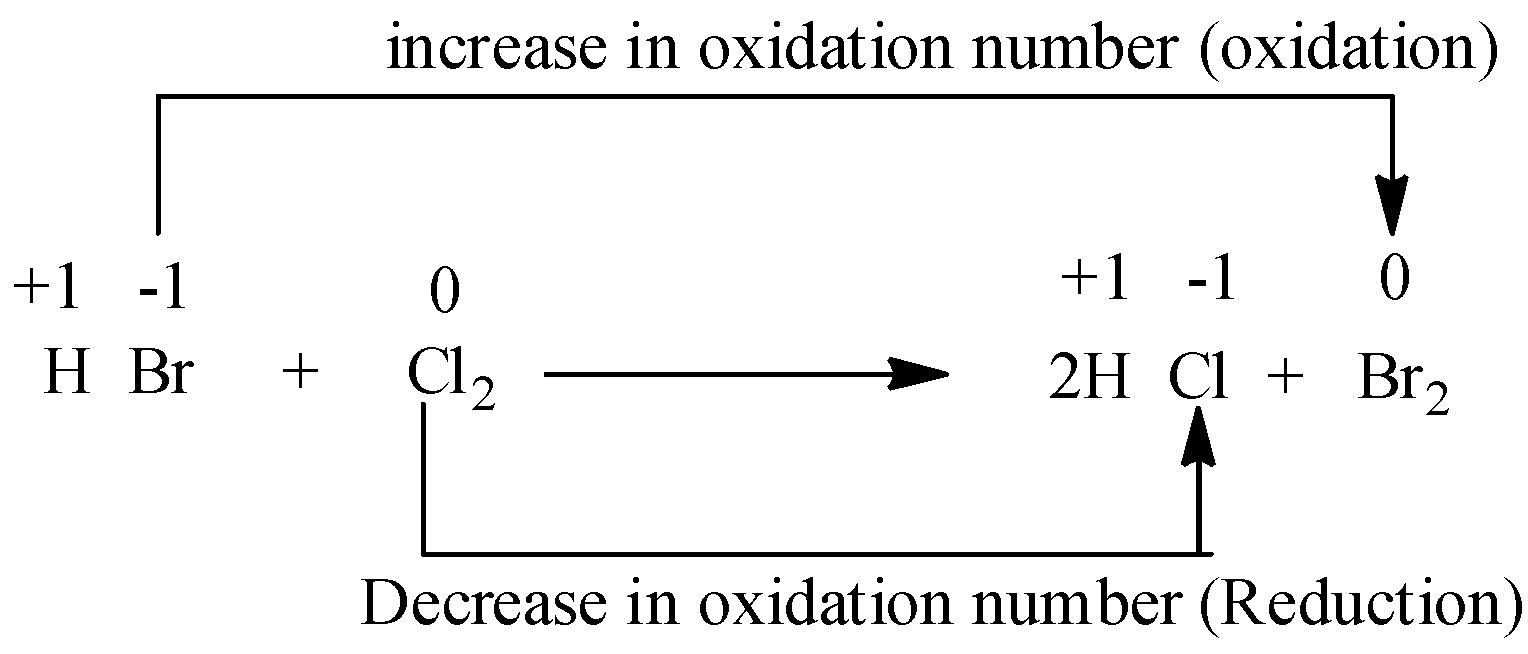
In the above reaction, the oxidation number of H is +1, Br is -1 and chlorine is 0 (reactant side) and in the product side oxidation number of H is +1, Cl is -1 and bromine molecule is 0. So, we observe an increase in oxidation number in case of bromine and decrease of oxidation number in case of chlorine. As both the oxidation and the reduction takes place, the reaction is a redox reaction.
Example 2)
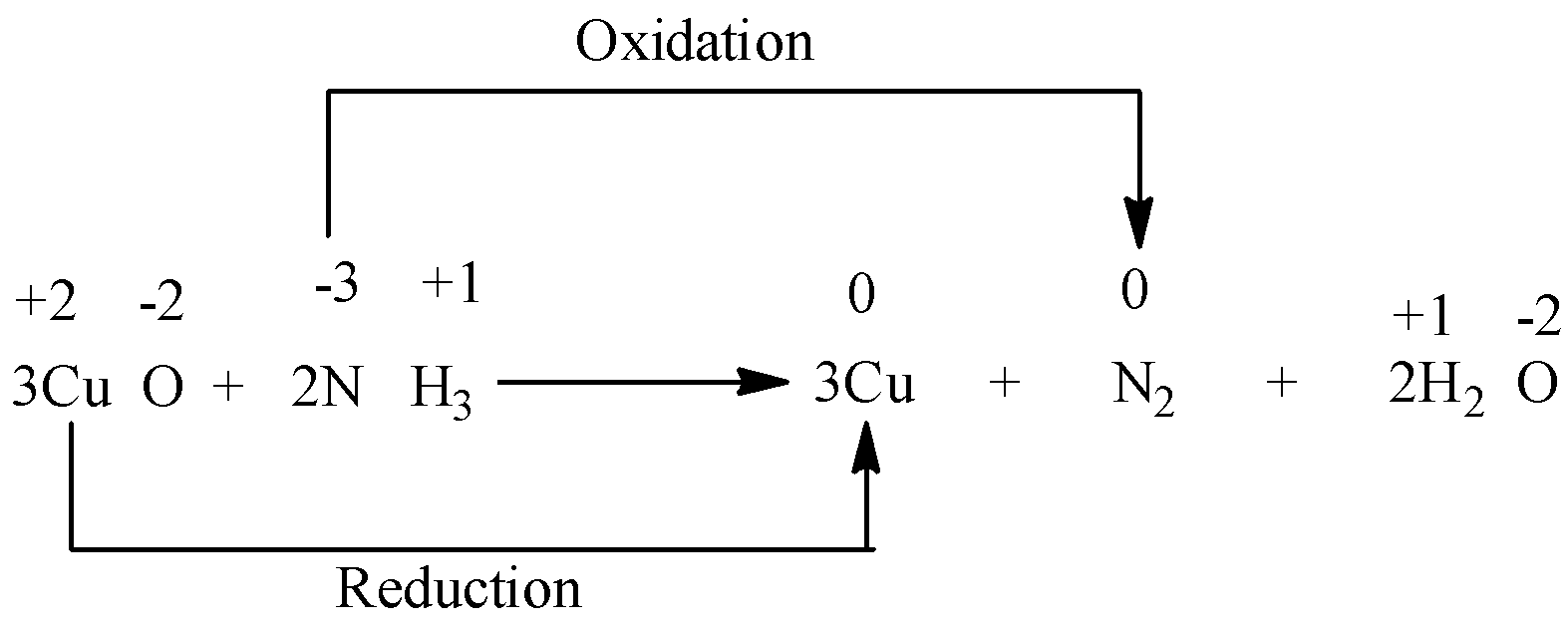
In the above reaction, we see that the oxidation number of nitrogen increases from -3 to 0 and oxidation number of copper decreases from +2 to 0. As both the oxidation and the reduction takes place, the reaction is a redox reaction.
Note: Always remember that, oxidizing agents are the substances that undergo decrease in oxidation number by gaining electrons and reducing agents are the substances that undergo increase in oxidation number by losing electrons.
