Question
Question: Give the structure of polyisoprene (natural rubber). Write down the ozonolysis of isoprene....
Give the structure of polyisoprene (natural rubber). Write down the ozonolysis of isoprene.
Solution
This question gives the knowledge about the natural rubber or polyisoprene and ozonolysis. Natural rubber is basically the polymer of isoprene units which are loosely attached that is why it is known as polyisoprene.
Complete step-by-step answer: Generally, various is isoprene units connect with each other to form natural rubber. It is obtained from the latex sap of rubber plants. . Natural rubbers are different from synthetic rubbers because natural rubbers are obtained from plants whereas synthetic rubbers are synthesized in laboratories. Synthetic rubbers are generally synthesized from petro chemicals. It is very elastic in nature.
Natural rubber is used for various applications which are as follows:
1.Due to compressibility and high tensile strength, it is useful in various engineering applications such as drive couplings, anti-vibration mounts, springs, rubber bands, adhesives and bearings.
2.It is also used in making high-performance tires for buses, aircrafts and race cars because of its heat resistant nature and high tensile strength.
3.It is also used in making shoe soles, footballs and so forth.
The structure of polyisoprene (natural rubber) consists of various isoprene units attached together. This can also be synthesized with the help of Ziegler-Natta polymerization.
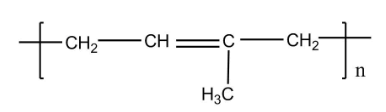
Ozonolysis is the process which leads to the cleavage of unsaturated bonds of alkynes, alkenes or azo compounds with the help of ozone.
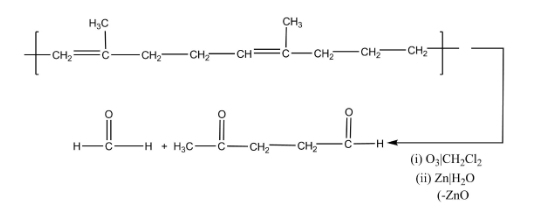
Note: Ozonolysis is the process which generally leads to the breakage of unsaturated bonds of alkynes, alkenes or azo compounds with the help of ozone molecules. Natural rubber is a polymer which is manufactured by the addition of various isoprene units.
