Question
Question: Give the structure of following: A) \[4 - tert.Butyl - 3 - iodoheptane\] B) \[1 - Bromo - 4 - s...
Give the structure of following:
A) 4−tert.Butyl−3−iodoheptane
B) 1−Bromo−4−sec.butyl−2−methyl benzene
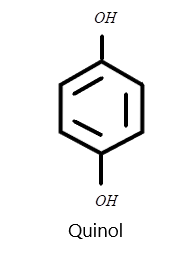
C) Quinol
D) Glycerol
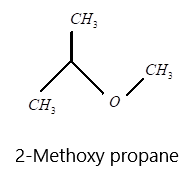
E) 2−methoxy propane
Solution
As we all know nomenclature involves two systems for naming of the organic compounds and these are trivial systems and IUPAC systems. IUPAC system consists of a root or base, primary and secondary prefix and suffix and any substituent for naming a compound.
Complete step-by-step answer:
As we have learnt that IUPAC is the short for International Union of Pure and Applied Chemistry. It provides some rules for naming of the organic compounds. Using these rules we can write a unique name for any distinct compound and likewise we can make the structure from the given IUPAC name. IUPAC system consists of three main features which are: a root representing the chain of carbon atoms in molecular structure, presence of primary prefix, secondary prefix, primary suffix and secondary suffix and lastly the name of the substituents completing the molecular structure or formula.
So let us try making structure with the help of these rules.
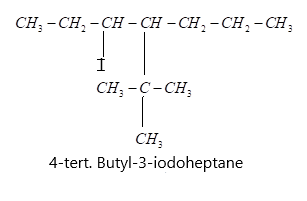
i) (a) 4−tert.Butyl−3−iodoheptane, in this formula the parent chain is heptane means 7 carbons are present in a chain.
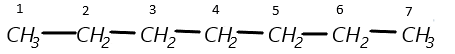
(b) Now, at the fourth carbon, a tertiary butyl is present so it can be represented as:
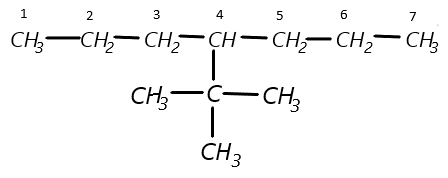
(c) Finally it is given that at third carbon an iodo-group is present so let us attach it and finally we get our desired structure:
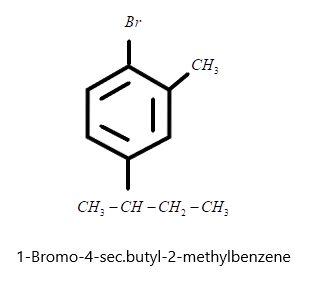
ii) 1−Bromo−4−sec.butyl−2−methylbenzene where the parent chain is benzene ring and bromine is present at its first carbon, methyl group at ortho position or 2ndcarbon and secondary butyl at para position or 4thcarbon. Hence we can make the structure simply as:
iii) Quinol structures basically have hydroxyl groups attached at the first and fourth carbon atom. So we can draw the structure easily as:
iv) Glycerol has the chemical formula as C3H8O3 so its structure can be drawn very easily as:
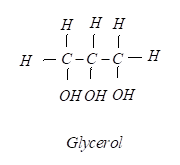
v) 2−Methoxypropane having propane as the parent chain and at second carbon methoxy group is present and the structure can be drawn as:
Note: Always remember that the parent chain will start from the side of attachment of the functional group. Also, while naming any compound put commas between numbers and dashes between letters and the numbers and while making structures first make the parent chain then add the substituents.
