Question
Question: Give the resonating structures of \[N{{O}_{2}}\]and \[{{N}_{2}}{{O}_{5}}\]...
Give the resonating structures of NO2and N2O5
Solution
We know the meaning of resonating structure. From this we have to draw the all delocalization of lone pairs, negative and positive charge etc.
Complete step by step solution:
We know that resonance is the way by which we describe delocalized electrons within certain molecules or polyatomic ions where the bonding can’t be expressed by a single Lewis formula or structure.
Resonance structures: These are the set of two or more Lewis Structures that collectively describe the electronic bonding of a single polyatomic species including partial bonds and partial charges.
Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integer number of covalent bonds.
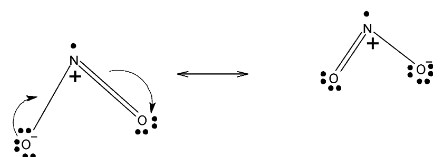
In case ofNO2,it has one single bonded oxygen and one double bonded oxygen. Or we can say that this double bond delocalized. Below given are resonating structures of NO2
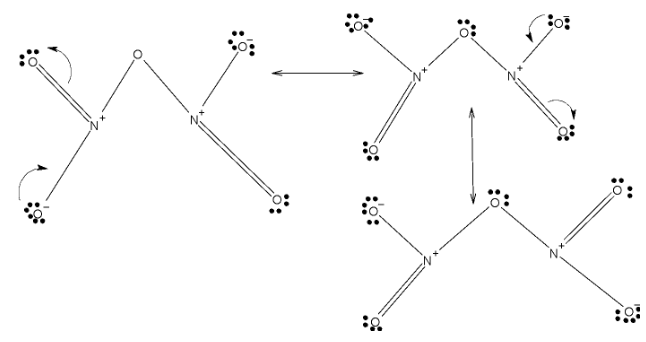
In case ofN2O5, it has two double bonded oxygens and one bridge oxygen and two single bonded oxygen. These two double bonds are localized among four oxygens. Below given are resonating structures of N2O5
So, the NO2has two resonating structures and N2O5 has 3 resonating structures
Note: So, while drawing resonating structures you should be careful about lone pairs and charges. Here all double bonds partially exist. And oxygen has partial negative charge.