Question
Question: Give the product of the oxidation with \(Mn{{O}_{2}}\) of \({{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\)....
Give the product of the oxidation with MnO2 of C6H5CH2OH.
Solution
The given reactant is aromatic alcohol whose name is Benzyl alcohol. The given oxidizing agent is Manganese dioxide, so the oxidation will occur, in which either there is the addition of oxygen atoms or there will be the removal of hydrogen atoms. Since it is a mild oxidizing agent, there will be the removal of hydrogen atoms.
Complete answer:
The given reactant is aromatic alcohol whose name is Benzyl alcohol having formula C6H5CH2OHand it is a member of benzylic alcohol because the carbon atom having the hydroxyl group is attached with the carbon atom of the benzene ring.
The given oxidizing agent is Manganese dioxide, so the oxidation will occur, in which either there is the addition of oxygen atoms or there will be the removal of hydrogen atoms. As allylic and benzylic alcohols easily get oxidized in mild conditions, where the primary allylic or benzylic alcohols give aldehydes while the secondary allylic or benzylic alcohols give ketones.
The given reactant Benzyl alcohol is a primary compound so it will give aldehyde. So, when the Benzyl alcohol reacts with manganese dioxide, it forms benzaldehyde. The formula of benzaldehyde is C6H5CHO. The reaction is given below:
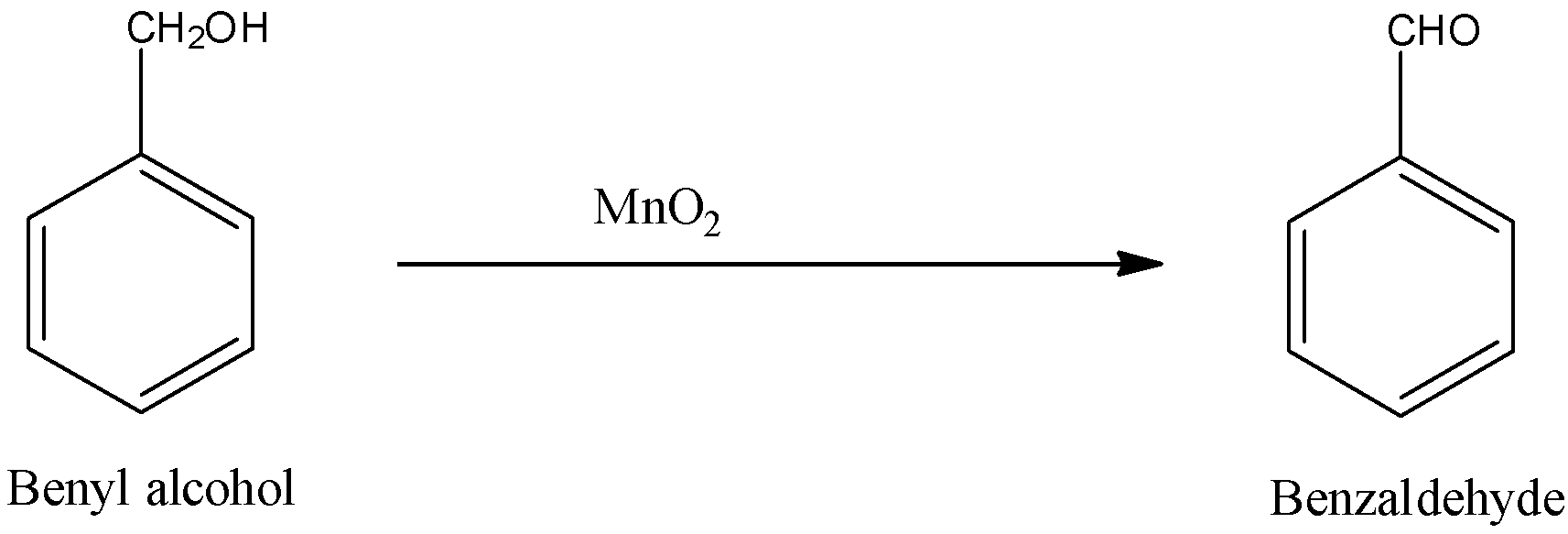
Therefore, the answer for the oxidation of C6H5CH2OH with MnO2 will be C6H5CHO.
Note:
When the allylic or benzylic alcohols are treated with manganese dioxide, then there should be an inert solvent like carbon tetrachloride, etc because this will prevent the attack of the double or triple bond.
