Question
Question: Give the product of oxidation with \(Mn{{O}_{2}}\)of \({{C}_{6}}{{H}_{5}}CH(OH)C{{H}_{2}}C{{H}_{2}}O...
Give the product of oxidation with MnO2of C6H5CH(OH)CH2CH2OH.
Solution
As we know that allylic and benzylic alcohol can be readily oxidized under mild conditions. Also manganese (IV) dioxide is a mild oxidizing agent which selectively oxidizes primary or secondary allylic alcohols. We will use this information of reagent in the given question.
Complete answer:
Let us first understand the oxidation of allylic and benzylic alcohol with the help of manganese (IV) dioxide:-
-The allylic and benzylic alcohols are oxidized specifically and selectively by a suspension of activated manganese (IV) dioxide (MnO2). Aldehydes are formed by the oxidation of primary allylic alcohols and ketones are formed by the oxidation of secondary allylic alcohols.
-For this reaction, we require an “activatedMnO2” which is obtained by the oxidation – reduction reaction of potassium permanganate and it is also a mild oxidizing agent. The oxidation of alcohols takes place on the surface of manganese (IV) dioxide (MnO2) which is insoluble in the solvents used for this reaction.
-The reaction is highly chemoselective because allylic and benzylic alcohols react more rapidly than the ordinary alcohols.
The oxidation reaction of C6H5CH(OH)CH2CH2OHwith the help of MnO2 is shown below:-
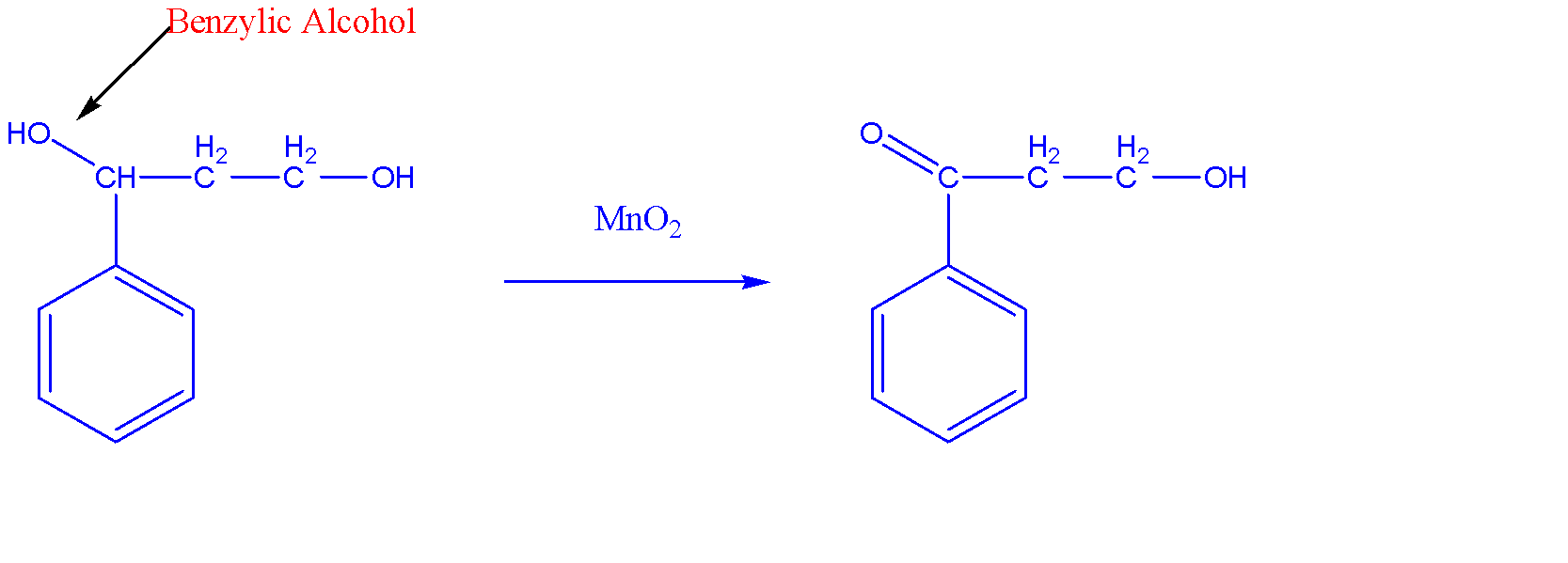
We can see that there are two alcohols in the reactant among which MnO2have selective nature towards benzylic alcohol and hence it will get oxidized to become a carbonyl compound. Since it is a secondary benzylic alcohol, we obtain ketone as a product.
Note:
The other reagents that can be specially used to oxidize allylic and benzylic alcohol are Mn(OAc)2and Mn(OAc)3.
-Remember that water competes with the alcohol for the sites on the MnO2surface and therefore it must be removed by drying to produce an active oxidant.
