Question
Question: Give the major product of the following reaction: ...
Give the major product of the following reaction:
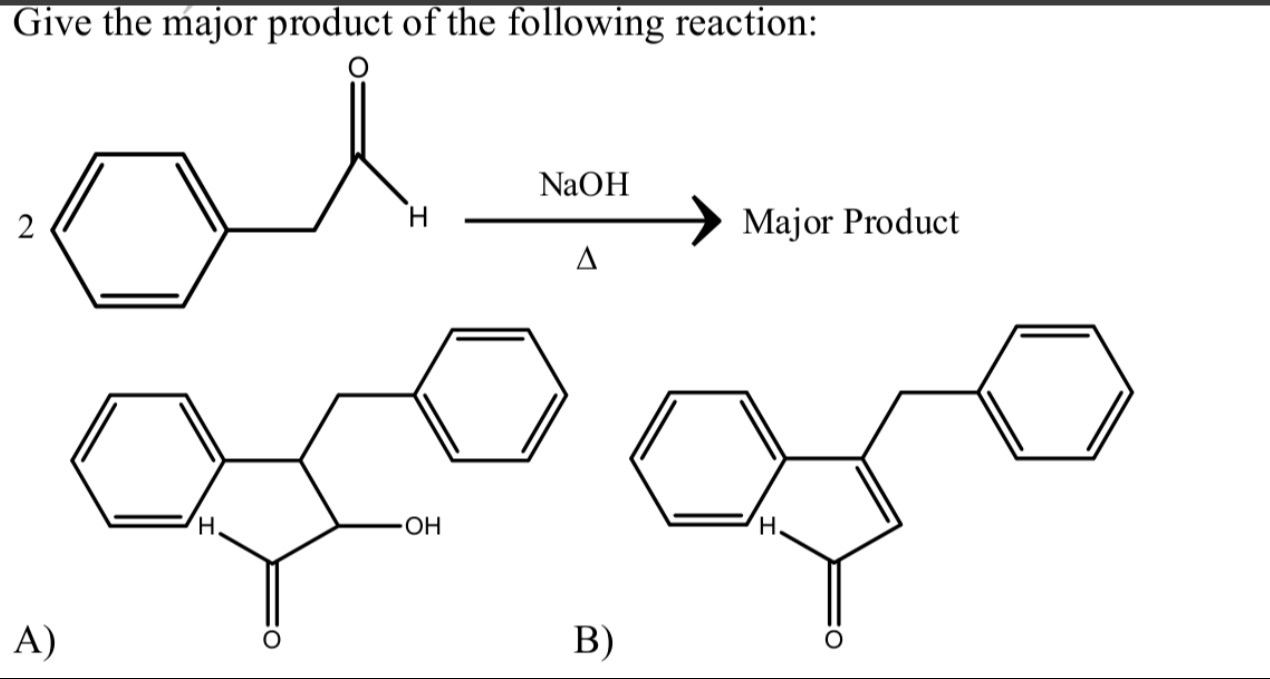
A
3-hydroxy-2-benzyl-2,3-dihydro-1H-inden-1-one
B
2-benzyl-1H-inden-1-one
Answer
B
Explanation
Solution
The reaction involves two molecules of phenylacetaldehyde undergoing an aldol condensation, followed by an intramolecular cyclization and dehydration.
- Intermolecular Aldol Condensation: Two molecules of phenylacetaldehyde (C₆H₅-CH₂-CHO) react. One molecule forms an enolate (C₆H₅-CH⁻-CHO) which attacks the carbonyl carbon of the second molecule. This yields the β-hydroxy aldehyde, 3-hydroxy-2,4-diphenylbutanal.
- Intramolecular Cyclization and Dehydration: Under heating (Δ), the aldol product undergoes dehydration to form an α,β-unsaturated aldehyde (2-phenyl-4-phenyl-2-butenal). This intermediate then undergoes an intramolecular electrophilic aromatic substitution (similar to Friedel-Crafts acylation) where one of the phenyl rings attacks the carbonyl carbon, forming a five-membered ring fused to the benzene ring. Subsequent dehydration (if needed) leads to the highly conjugated 2-benzyl-1H-inden-1-one (Option B). Option A is the β-hydroxy intermediate which would dehydrate to form B under heating conditions. Therefore, B is the major product.
