Question
Question: Give the major product of the following reaction. 
(a) 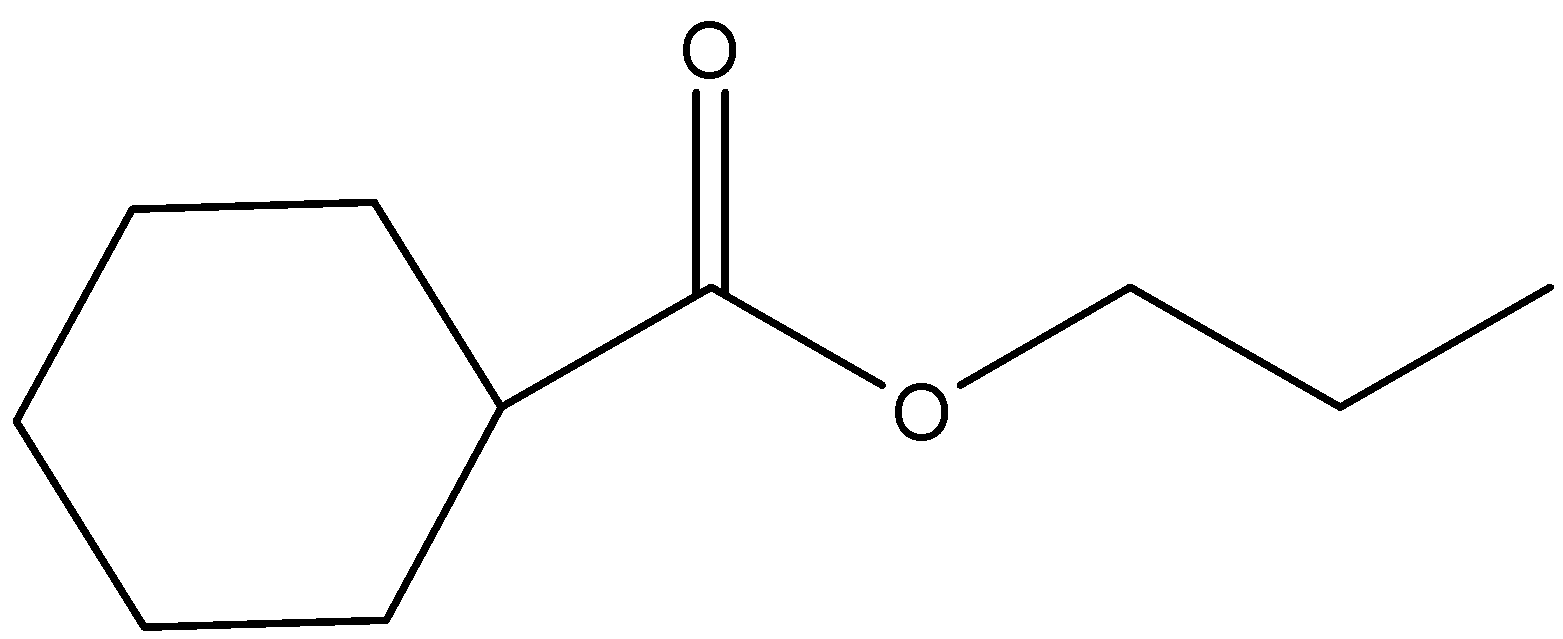
(b) 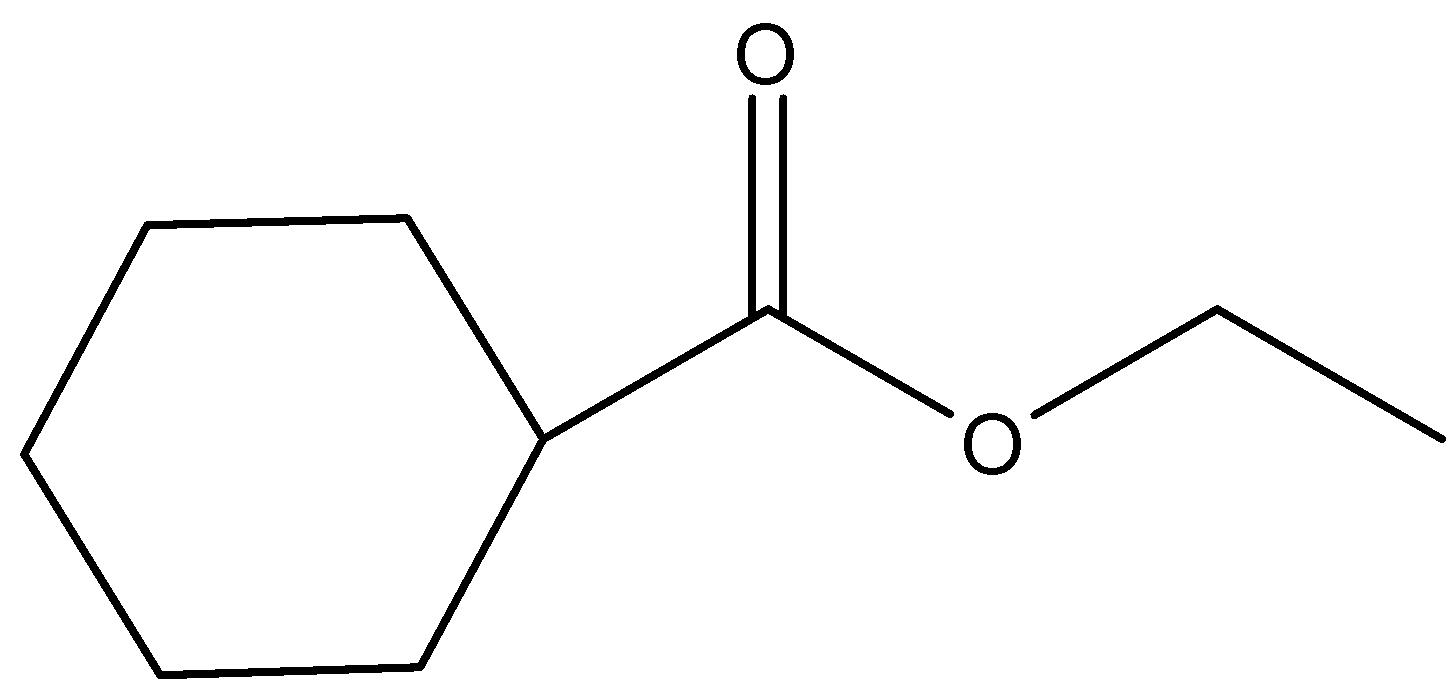
(c) 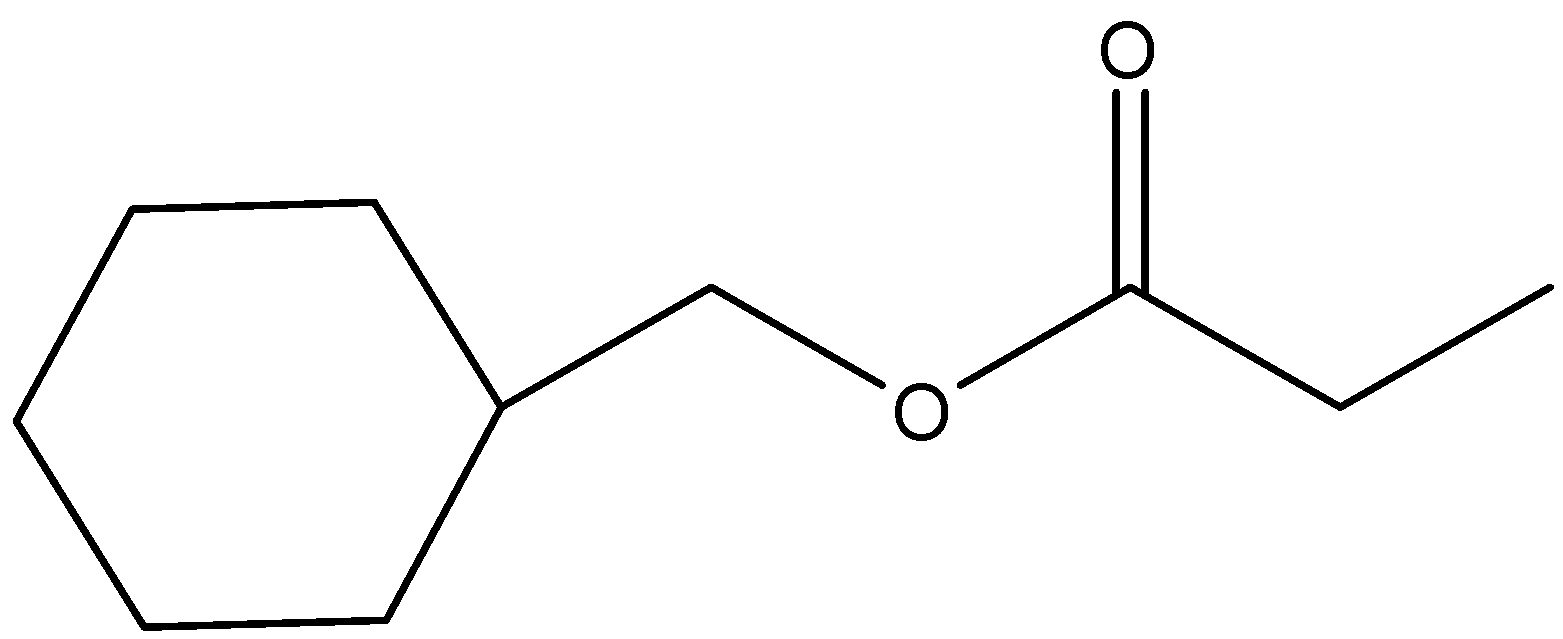
(d) 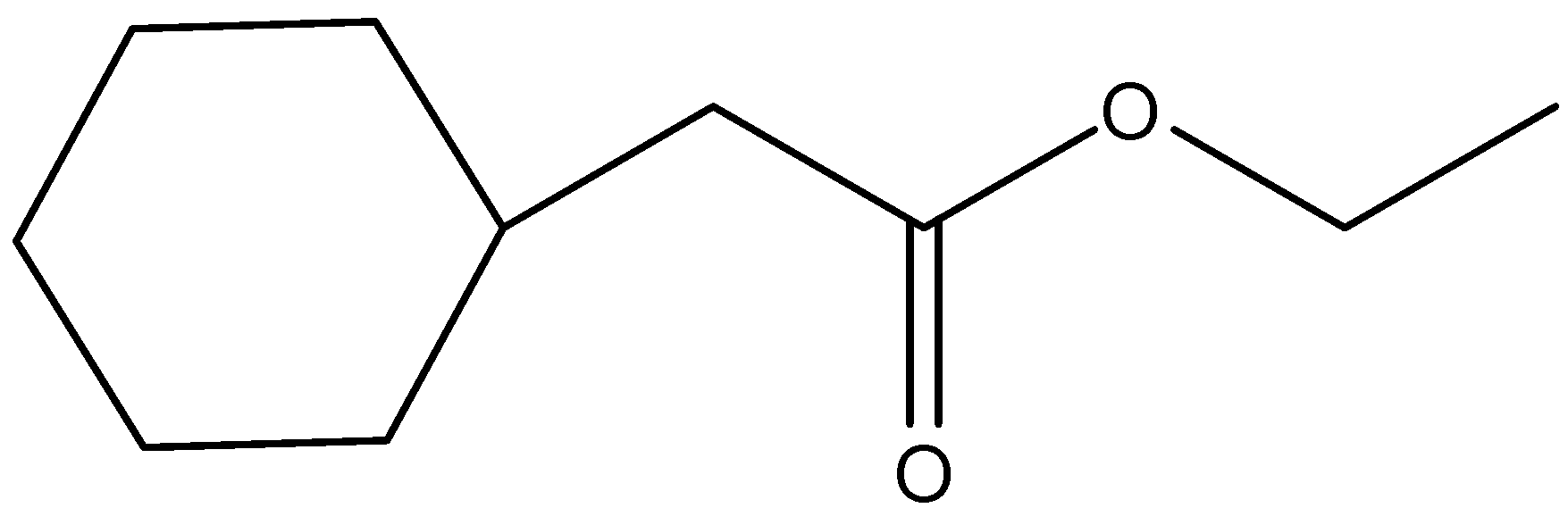
Solution
There are a series of reactions given in the question. We need to predict each reaction product, depending on the reagent used. The first reagent used here is a reducing agent. So, the product will be a reduced product,which will be an alcohol. Another reagent is an ester, so its corresponding product formed. This reaction is called transesterification reaction.
Complete step by step answer:
- In the question, we can see that an ester (IUPAC name is cyclohexyl acetate) is given. It is reacted with Lithium aluminium hydride and the product formed is further reacted with another ester to give the final product. Let us consider the reaction step wise.
-First step: The reagent used here is lithium aluminium hydride that means reduction of the ester is occurring to give a primary alcohol, that is cyclohexyl methanol. The reaction can be written as following
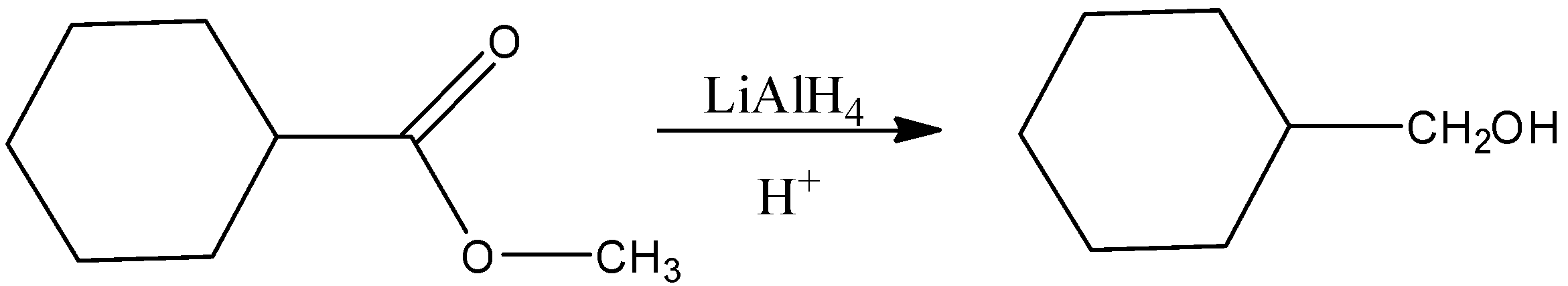
-Second step: This primary alcohol is then reacted with another ester (ethyl propanoate) to give another ester (ethyl cyclohexyl acetate). This reaction is called transesterification. Esterification is a reaction where an alcohol and an acid react in the presence of concentrated sulphuric acid and heat to give a sweet smelling compound called esters and transesterification process is the exchange of the R group of an ester with the R’ group of an alcohol. In this reaction R group of ester is CH3CH2∣C=O and the R’ group of alcohol is.
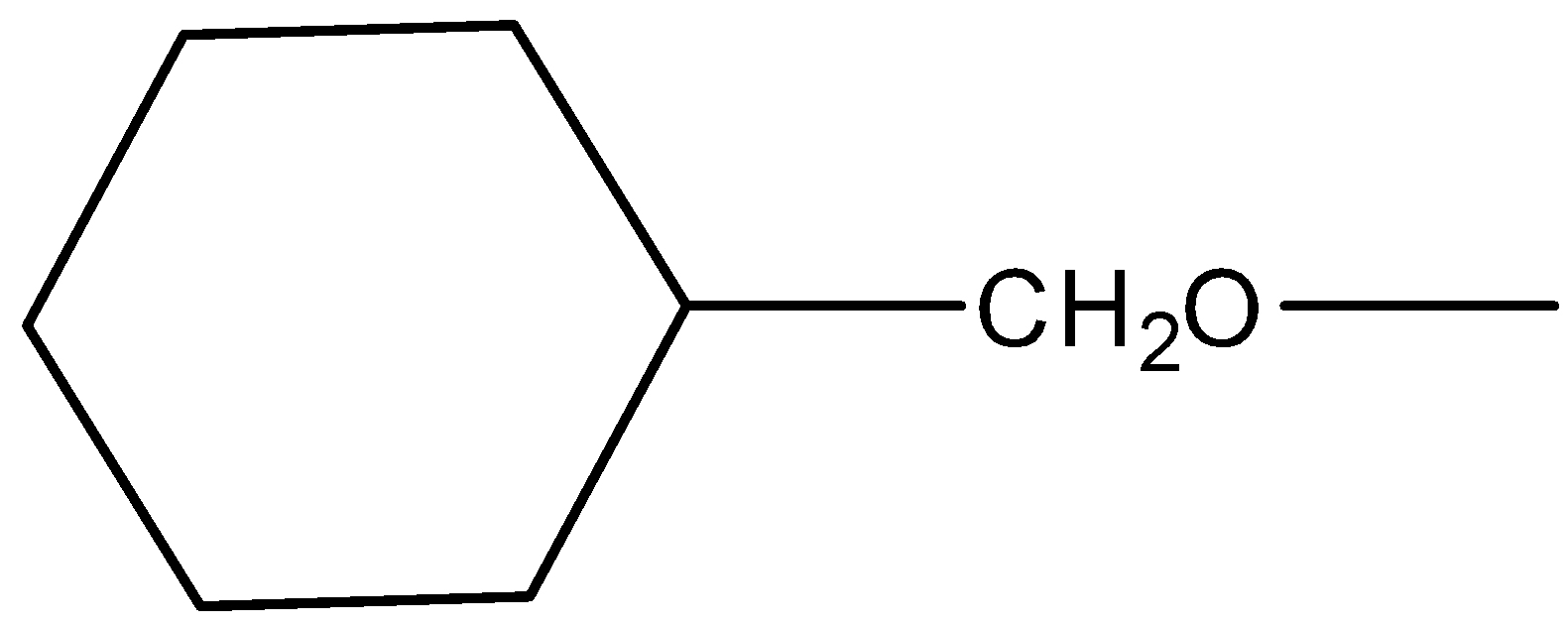
So, the reaction is as follows:
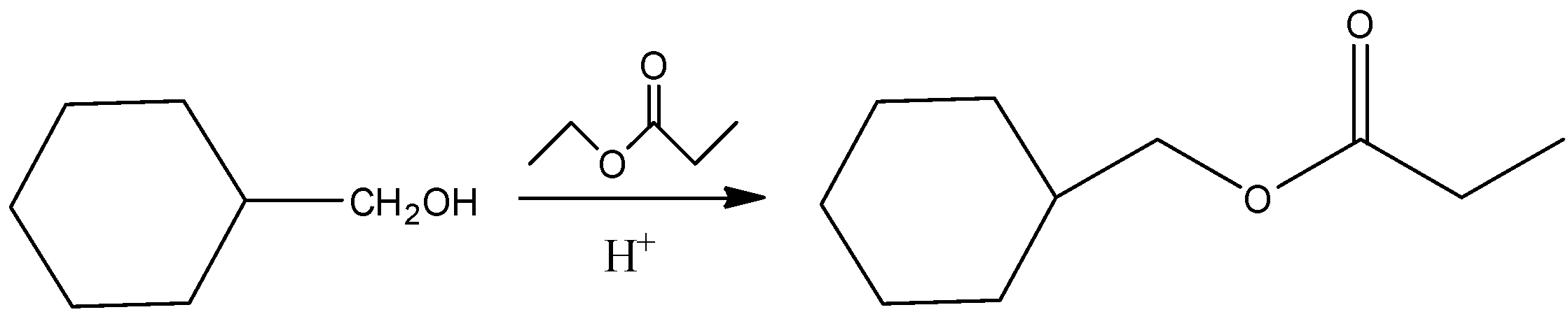
So, the correct answer is “Option C”.
Note: We should note that here in this conversion trans product is obtained. It is due to the exchange of the alkyl group. Cis product is not formed in such reactions.
