Question
Question: Give the IUPAC name of the above compound: 
Solution
The method of naming organic compounds is known as the IUPAC nomenclature. In the given structure, a double bond and a halogen atom are present. First we need to find the parent chain. Let us discuss it step by step.
Complete step by step answer:
The given structure with all of its atoms is as follows:
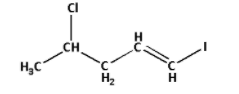
Select the longest continuous chain of carbon atoms containing carbon-carbon double bonds in the structure of the molecule.
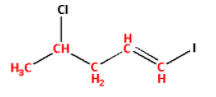
Number the carbon atoms in the selected carbon chain from the end which is nearest to the double bond.
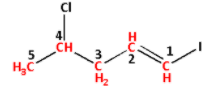
The longest chain contains five carbon atoms. Thus, the parent alkane is pentane.
Replace suffix ‘ane’ of propane by ‘ene’. Thus, the parent alkene is pentene. The double bond is attached to carbon number 1. Thus, pent-1-ene.
The chlorine group is attached to carbon number 4. Thus, 4-chloro.
The iodine group is attached to carbon number 1. Thus, 1-iodo.
Write the names of the groups in alphabetical order.
Thus, the IUPAC name is 4-chloro-1-iodopent-1-ene.
Note: The rules for writing IUPAC name of alkenes are as follows:
- Select the longest continuous chain of carbon atoms containing carbon-carbon double bonds in the structure of the molecule.
- Number the carbon atoms in the selected carbon chain from the end which is nearest to the double bond.
- Count the number of carbon atoms in the chain. This is the parent alkane.
- The name of alkene is written by replacing suffix ‘ane’ in the parent alkane by ‘ene’
- Write the number indicating the position of the double position before prefix ‘ene’.
- Assign a number to each substituent according to the carbon atom it is attached to. If there are two substituents on the same carbon, assign the same number to them.
