Question
Question: Give the IUPAC name for the following structures: 
A. 2-methyl-5-chlorocyclohexanol
B. 2-chloro-4-methylcyclohexanol
C. 5-chloro-2-methylcyclohexanol
D. 3-chloro-2-methylcyclohexanol
Solution
We need to remember that the alcohols are organic compounds that contain hydroxyl groups. We can write the general formula of alcohols as R−OH , here R is the alkyl group. Aldehydes are used in the preparation of plasticizers and polyols, they could be reduced to alcohols. Primary alcohols, secondary alcohols, and tertiary alcohols are the three types of alcohols.
Complete step by step answer:
Now we can discuss about the IUPAC naming of alcohol as,
1.Locate the longest chain containing the hydroxyl group (OH) . On the off chance that there is a chain with a larger number of carbons than the one containing the OH bunch it will be named as a substituent.
2.Spot the OH on the most minimal conceivable number for the chain.
3.When naming a cyclic structure, the −OH is thought to be on the main carbon except if the carbonyl group is available, in which case the later will get needed at the primary carbon.
4.At the point when numerous −OH bunches are on the cyclic structure, number the carbons on which −OH bunches live.
5.Eliminate the last e from the parent alkane chain and add - ol. At the point when different alcohols are available use di, tri, etc before the ol, after the parent name. ex. 2,3-hexandiol. In the event that a carbonyl group is available, the −OH bunch is named with the prefix "hydroxy," with the carbonyl group appended to the parent chain name so it closes with - al or - one.
The given compound is,
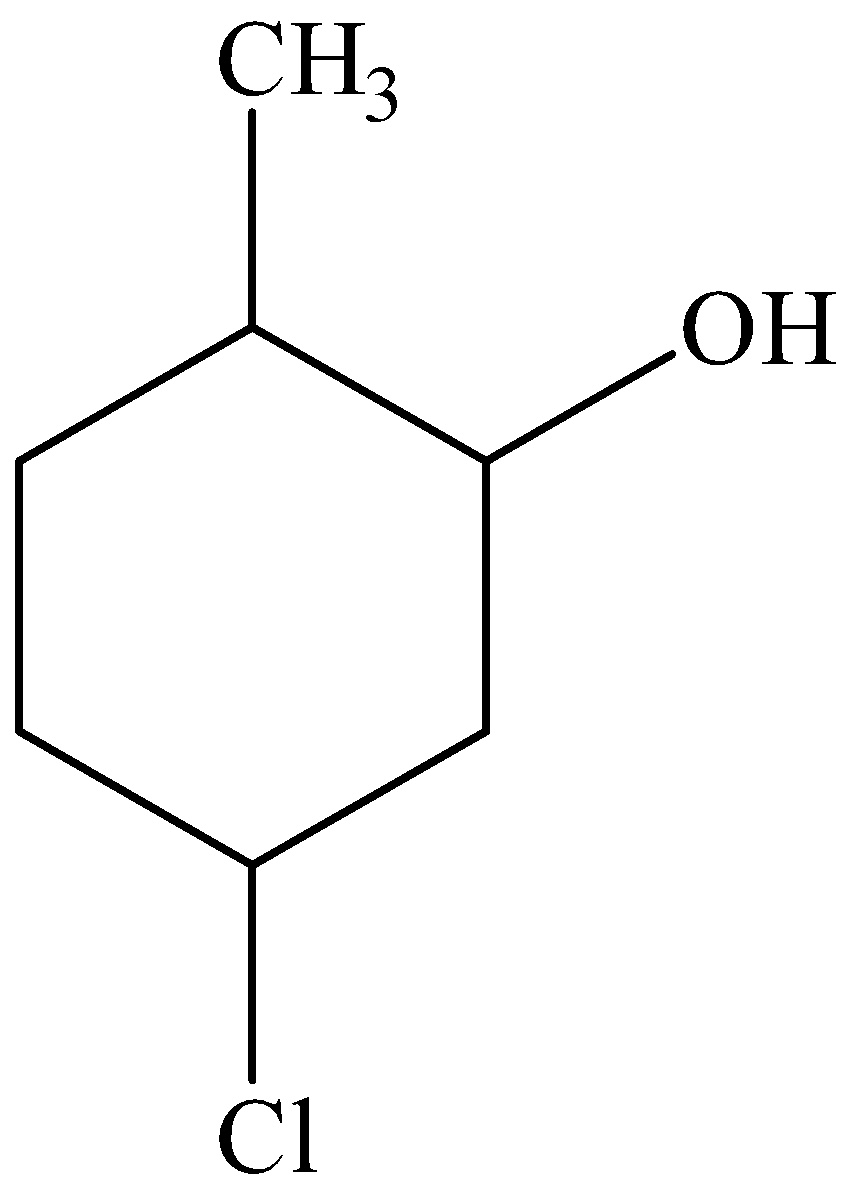
We must know that in the cyclohexane ring, the presence of hydroxyl group in the ring gives the parent compound name as cyclohexanol. In the second position, a methyl group is present and in the fifth position, a chloro substituent is present in the cyclohexane ring. The IUPAC name of the compound is 5-chloro-2-methylcyclohexanol.
So, the correct answer is Option C.
Note: We must have to remember that the alcohols possess strong intermolecular hydrogen bonding. The OH group present in the alcohol permits the molecules to join in hydrogen bonding. A larger amount of energy is desirable to break the intermolecular hydrogen bonding in alcohols hence; they comprise higher boiling and higher melting points when compared to hydrocarbons of comparable molar mass.
