Question
Question: Give the formula of each of the following coordination entities: (i) \({\text{C}}{{\text{o}}^{{\te...
Give the formula of each of the following coordination entities:
(i) Co3 + Ion is bound one Cl−, one NH3 and two bidentate ethylenediamine (en) molecule.
(ii) Ni2 + Ion is bound to two water molecules and two oxalate ion
Write the name and magnetic behaviour of each of the above coordination entities.
(At. Co = 27, Ni = 28)
Solution
To write the formula, write the symbol of metal followed by the symbol of all ligands. Name of compounds is written by using the IUPAC rules and the presence of unpaired electrons in the metal complex tells the paramagnetic nature of the complex.
Complete step by step answer:
Write the name of the cation part then the name of the anion part.
Write the name of the ligand in alphabetical order.
For neutral ligand, the name of the compound is used as such but for water use ‘aqua’ for ammonia use ‘ammine’
For anionic ligands replace the,
Ide with o.
Ate with ato.
Ite with ito.
Use Greek prefix to denote the number of the ligand. If the name of the ligand has a Greek prefix then use bis for two, tris for three.
Write the name of the metal.
For the cationic complex, write the metal name as such.
For anionic complex, use the suffix ‘ate’.
(i) The coordination entity of cobalt metal has one chloride, one ammonia and two ethylenediamine ligands. The denticity of ethylene diamine (en) ligand is two.
So, the coordination of cobalt metal six.
So, the coordination entity of cobalt metal is [CoClNH3(en)2] .
Determine the charge of the coordination entity as follows:
Suppose the charge of the coordination entity is x. Charge of cobalt ion is +3, charge of chloride is −1 and charge of ammonia and en ligand is zero.
x=+3+(−1)+0+(2×0)
x=+2
So, the charge on the coordination entity is +2.
So, the formula of the coordination entity is [CoClNH3(en)2]2+.
The name of the [CoClNH3(en)2]2+ compound is is as follows:
Chloroamminebisethylenediamminecobalt(II).
(ii) The coordination entity of nickel metal has two water and two oxalate ion. The denticity of oxalate ligands is two.
So, the coordination of nickel metal is six.
So, the coordination entity of nickel metal is [Ni(H2O)2(ox)2] .
Determine the charge of the coordination entity as follows:
Suppose the charge of the coordination entity is x. Charge of nickel ions is +2, charge of oxalate ions is −2 and water ligands are zero.
x=(+2×1)+(0×2)+(−2×2)
x=+2
So, the charge on the coordination entity is +2.
So, the formula of the coordination entity is [Ni(H2O)2(ox)2]2+.
The name of the [Ni(H2O)2(ox)2]2+ compound is is as follows:
diaqua dioxalato nickel (II).
The magnetic property of each complex is as follows:
The splitting of d-orbital in presence of ligand field and filling of electrons is represented as follows:
Valence electronic configuration of Co3+=3d6.
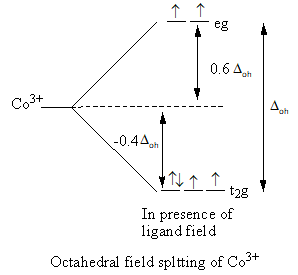
[CoClNH3(en)2]
The cobalt ion has four unpaired electrons so the complex is paramagnetic.
Valence electronic configuration of Co3+=3d6.
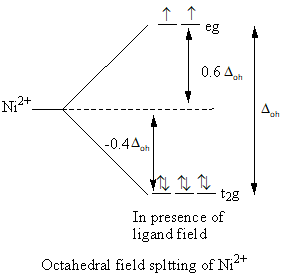
The nickel ion has two unpaired electrons so, the complex [Ni(H2O)2(ox)2] is paramagnetic.
Therefore, the formula of coordination entities are [CoClNH3(en)2]and [Ni(H2O)2(ox)2]. The name of cobalt complex is Chloroamminebisethylenediamminecobalt (II) and nickel complex is diaqua dioxalato nickel (II). Both cobalt and nickel complexes are paramagnetic.
Note:
The molecules that bind with the central atom are known as ligands. Denticity defines the number of donor atoms in a ligand. The d-orbitals of the metal remain degenerate in absence of ligand field and split in presence of ligand field.
