Question
Question: Give the definition of acid anhydride....
Give the definition of acid anhydride.
Solution
A corrosive is an atom or particle equipped for giving a proton (hydrogen particle H+) (a Bronsted–Lowry corrosive), or, then again, fit for shaping a covalent bond with an electron pair (a Lewis corrosive). The primary classification of acids is the proton givers, or Bronsted–Lowry acids.
Complete step by step answer:
A corrosive anhydride is a sort of synthetic compound got from the expulsion of water particles from a corrosive. Inorganic science, natural corrosive anhydrides contain the useful gatheringR(CO)O(CO)R′. Natural corrosive anhydrides regularly structure when one likeness water is eliminated from two reciprocals of a natural corrosive in a drying out response. In inorganic science, a corrosive anhydride alludes to an acidic oxide, an oxide that responds with water to frame an oxyacid (an inorganic corrosive that contains oxygen or carbonic corrosive), or with a base to shape a salt.
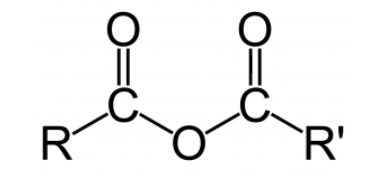
Nomenclature:
The terminology of natural corrosive anhydrides is gotten from the names of the constituent carboxylic acids which went through parchedness to frame the compound. In balanced corrosive anhydrides, where just a single constituent carboxylic corrosive was utilized to shape the compound, (for example, the drying out of propanoic corrosive, 2CH3CH2COOH → CH3CH2C(O)OC(O)CH2CH3+ H2O)just the prefix of the first carboxylic corrosive is utilized and the addition "anhydride" is added. In lopsided corrosive anhydrides, where two distinctive carboxylic acids were utilized to give the anhydride (for instance, the drying out between benzoic corrosive and propanoic corrosive, C6H5COOH + CH3CH2COOH → C6H5C(O)OC(O)CH2CH3+ H2O), the prefixes from the two acids responded are recorded before the postfix, for this situation giving benzoic propanoic anhydride, which may then again be alluded to as benzene carboxylic ethanoic anhydride.
Note: It was first set up by French scientific expert Charles Frederic Gerhardt in 1852 through warming potassium acetic acid derivation with benzoyl chloride. Acidic anhydride is set up via carbonylation of methyl acetic acid derivation.
