Question
Question: Give the decreasing order of the reaction rates of the following benzyl alcohol with \(HBr\) 
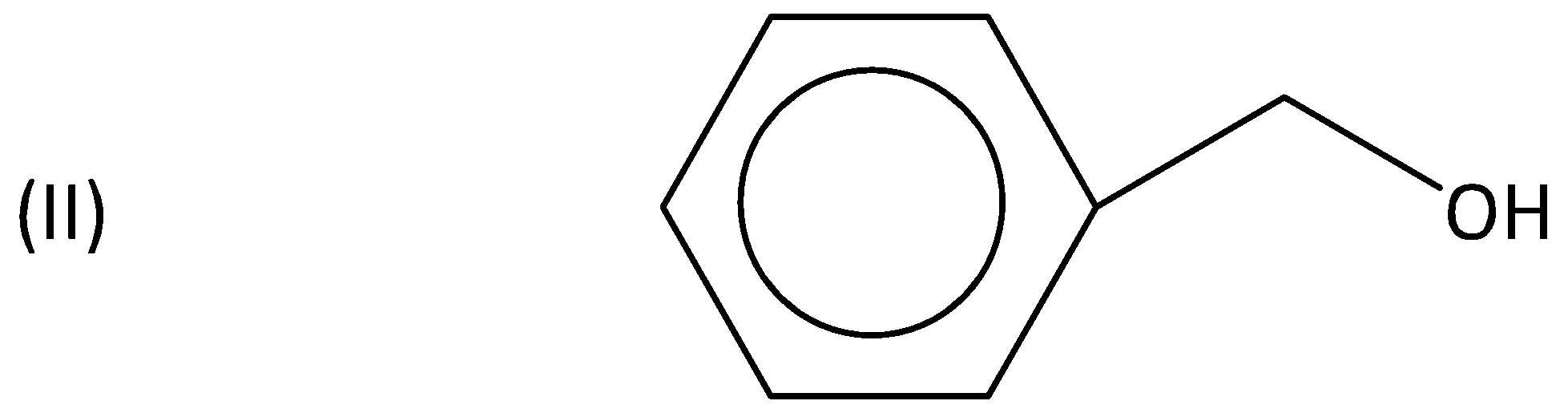
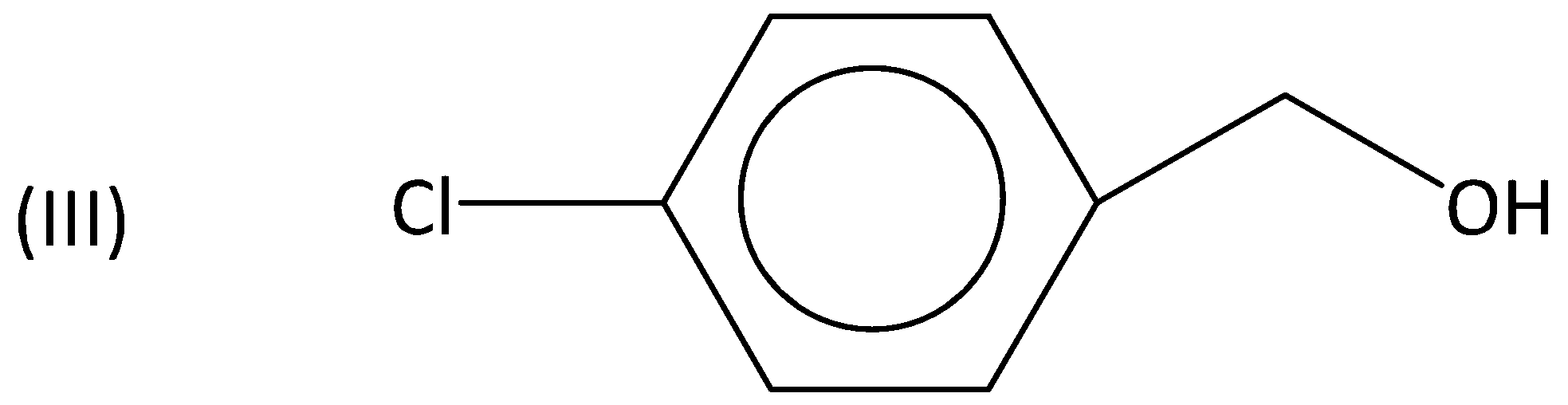
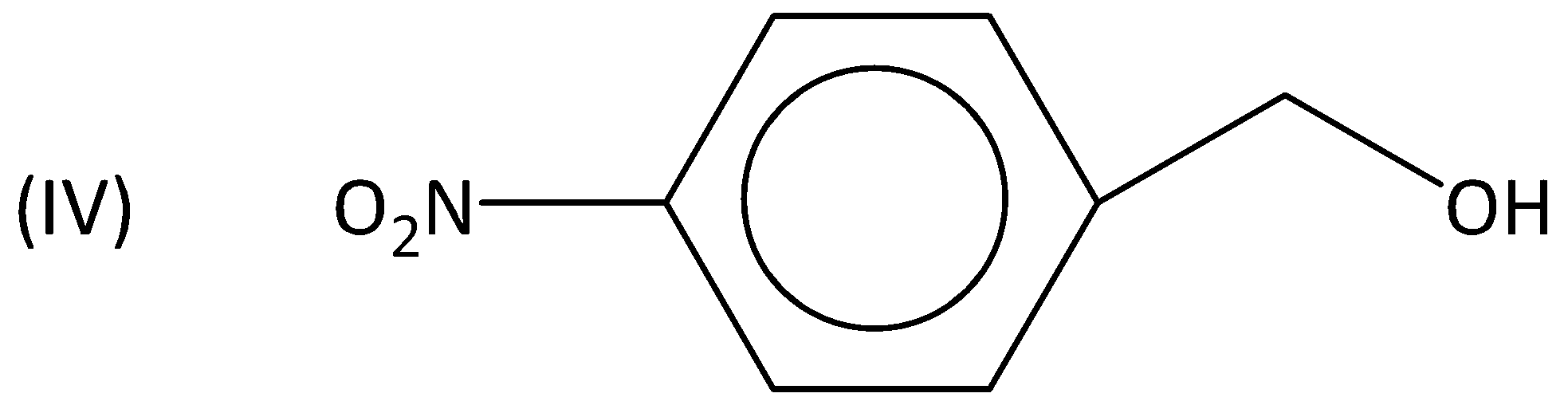
A.I>II>III>IV
B.IV>III>II>I
C.II>I>III>IV
D.None of these
Solution
We can predict the decreasing order of reaction rate of substituted benzyl alcohols with hydrogen bromide with the help of electron donating groups and electron releasing groups. We know that in case of substituted aromatic compounds, the functional group present in the compound leads the next incoming group to a particular position in the aromatic ring. We call this as the directive influence of the group already bonded to the benzene ring.
Complete step by step answer:
We must remember that if a mono substituted compound is treated with an electrophile, it would go through an electrophilic aromatic substitution reaction and produce a disubstituted compound which may be identified using the descriptors ortho, meta, and para. If the relative yield of the ortho product and the para products are more than that of the meta product, then the substituent found in the monosubstituted ring is labeled an ortho, para directing group.
Electron donating groups are usually ortho/para directors for electrophilic aromatic substitutions, whereas electron withdrawing groups are usually meta directors. The halogens are ortho/para directors as they require unshared pairs of electrons to share with the aromatic ring.
Electron donating groups are referred to as activating groups, though steric effects could inhibit the reaction. An electron withdrawing group has the opposite effect on the nucleophilicity of the ring. The electron withdrawing group takes away electron density from a π system, thereby making it less reactive during this kind of reaction and thus called as deactivating groups.
Generally activating groups’ increases the rate of the reaction and deactivating groups decreases the rate of the reaction.
So, we can give the decreasing order of the rates of reaction of the following benzyl alcohol with hydrogen bromide as follows,
When an electron donating group such as a methoxy group is present, an increased rate of reaction would be seen. Hence (I) is more reactive when compared to (II).
Whereas an electron withdrawing group such as nitro group (or) chloro group is present, they decrease the reactivity to a greater extent and therefore, the rate of the reaction is also less.
Therefore, (III) and (IV) are less reactive when compared to (I). So, the order is I>II>III>IV. Option (A) is correct.
Note:
We have to remember that electron donating groups are called as activating groups and electron withdrawing groups are deactivating groups. When electron density is added to the electron donating group, the pi system tends to become more nucleophilic, and less nucleophilicity is observed when electron density is removed from the pi system by the electron withdrawing group.
