Question
Question: Give structural formula for an isomer of n – butane....
Give structural formula for an isomer of n – butane.
Solution
In order to solve this question follow the following steps: first, find the molecular structure of the given compound using IUPAC rules for nomenclature. Then determine the types of atoms and the number of these atoms present in this compound. Finally, we must rearrange the positions of these atoms in such a way that they form a stable compound, wherein all the atoms their valencies satisfied.
Complete step by step answer:
Before we move forward with the solution of this question, let us understand a few basic concepts.
Isomerism can be understood as the phenomenon wherein a given set of compounds have the same number and the same types of atoms. This means that when we draw the molecular structures for these molecules, we use the same atoms in the same quantities but the arrangements of these atoms in the structures would be slightly or completely different.
Now let us get back to the question. The compound given to us n – butane. If we follow the rules of IUPAC nomenclature, then we can understand that the given compound is an aliphatic organic compound with 4 carbon atoms. To put this in simpler terms, it is basically an alkane with 4 carbon atoms. The structure of n – butane can be shown as:
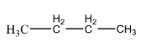
Now, as we can see, the molecular formula for this compound is C4H10 , i.e. there are 4 carbon atoms and 10 hydrogen atoms. An isomer for this compound can be represented as follows:
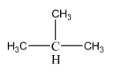
Using the IUPAC nomenclature rules, the name for this compound can be determined to be 2 – methyl propane.
Note:
The isomerism exhibited in this question is chain isomerism. There are other types of isomerism that exist as well, viz. position isomerism and functional group isomerism. Position isomerism occurs when a functional group is in a different position on the same carbon chain. Functional group isomerism, the isomers contain different functional groups.
