Question
Question: Give reason:\(NaCl\left( {aq} \right)\) Gives a white precipitate with \(AgN{O_3}\) solution but \(C...
Give reason:NaCl(aq) Gives a white precipitate with AgNO3 solution but CCl4 or CHCl3 does not because:
A) NaCl is a covalent compound and forms AgCl as white precipitate.
B) NaCl is an ionic compound and forms AgCl as white precipitate.
C) CCl4 and CHCl3 are ionic compounds.
D) None of these.
Solution
We know Ionic Bonding: Ionic bonding is the complete exchange of valence electrons between molecules. In ionic bonds the metal loses electrons to turn into a decidedly charged cation through the nonmetal acknowledges those electrons to turn into an adversely charged anion.
Covalent bonding: Covalent bonding is the sharing of electrons between particles. This sort of holding happens between two molecules of a similar component.
Complete step by step answer:
We have to remember that the sodium chloride being an ionic compound and along these lines reacts with Silver nitrate to give ionic compound thus silver chloride is precipitated as white precipitate. We can write the chemical equation for the formation of silver chloride precipitate as,
NaCl+AgNO3→AgCl+NaNO3
We must remember that the carbon tetrachloride and CHCl3 being covalent don't outfit chlorine particles in arrangement.
We have to remember that the sodium chloride is an ionic compound and water is a covalent compound. The melting points of compounds with ionic bonds have much higher melting point than covalently bonded molecules.
So, the correct answer is Option B.
Note: We can state octet rule, as "A molecule is steadier when their furthest shells are loaded up with eight electrons”. Molecules are not stable in single structure, aside from respectable gases as their furthest electrons are totally filled. To be steady, molecules consolidate with one another.
Now let us see the formation of sodium chloride due to transfer of electrons,
Sodium chloride is an ionic compound and so the formation takes place due to transfer of electrons from sodium to oxygen atom.
We can draw the Lewis structure of Sodium chloride,
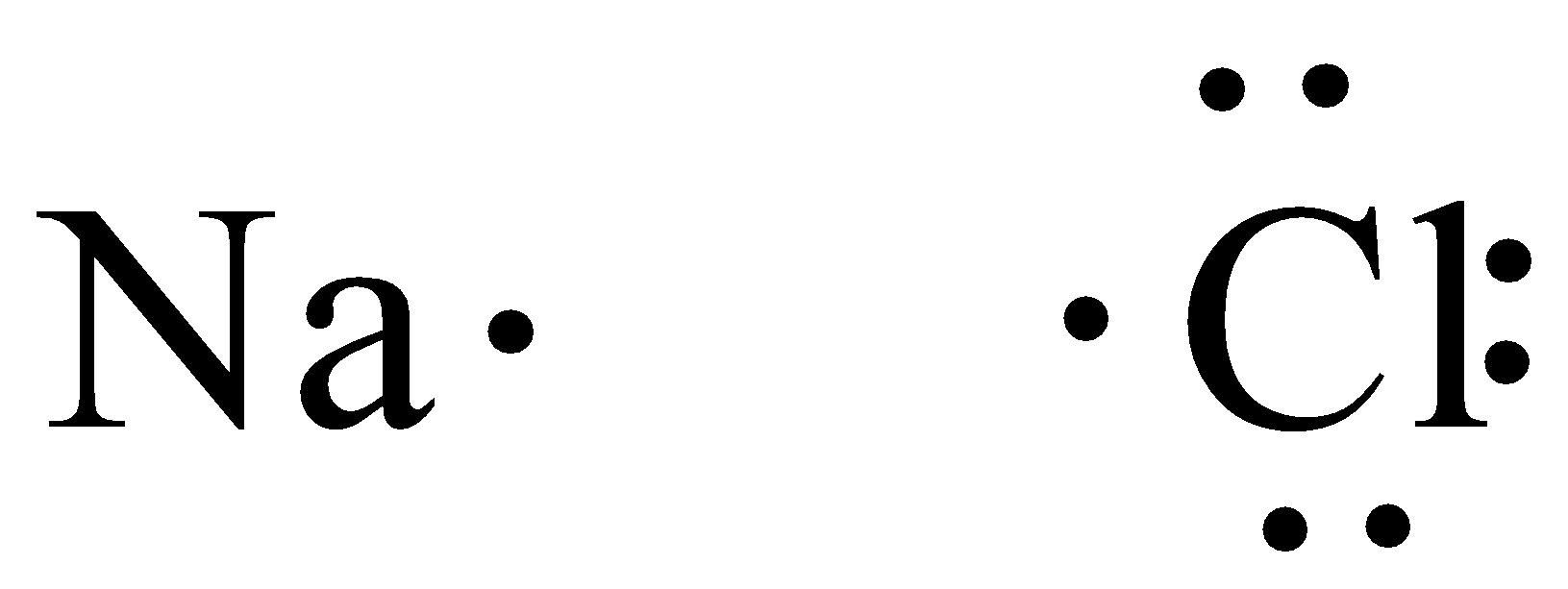
From the Lewis structure, we can see that chlorine needs one electron to have filled valence orbitals. Sodium moves one electron to chlorine since it needs one electron to satisfy its octet.
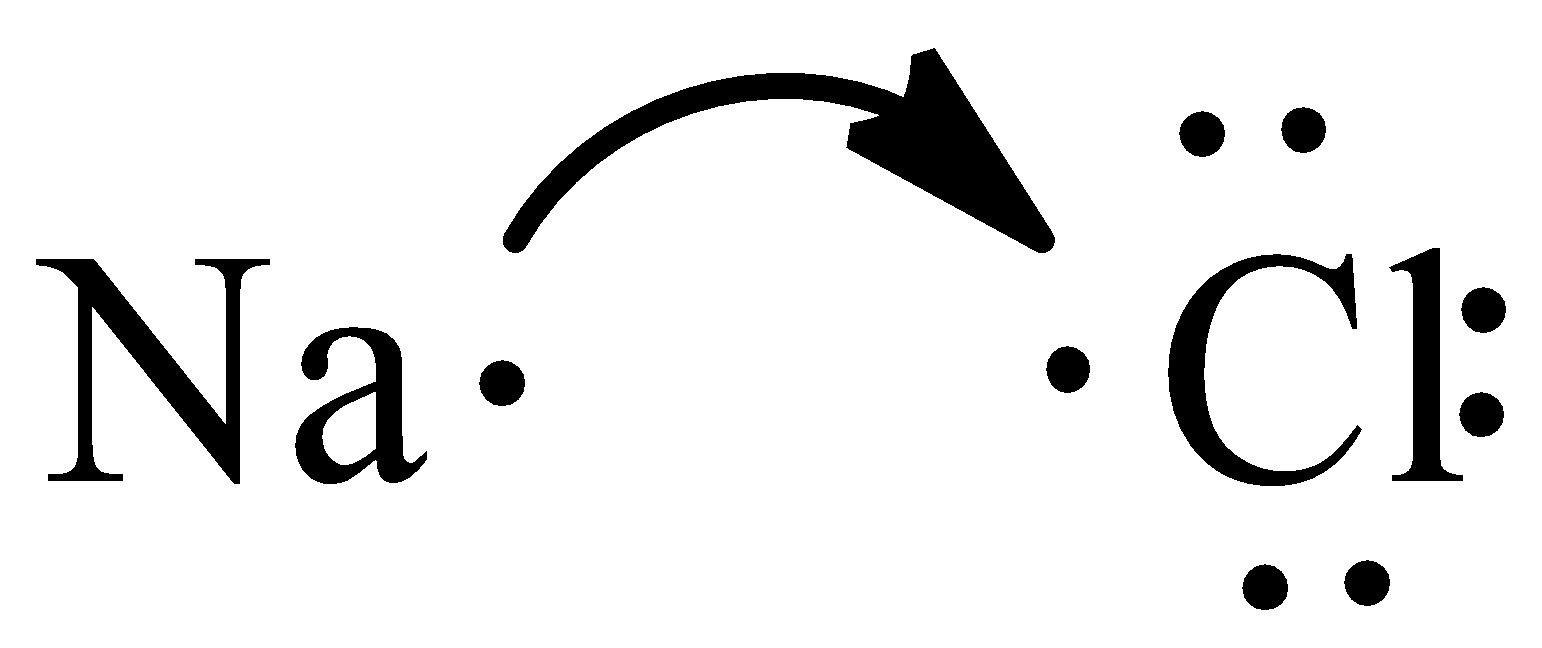
The general impartial charge ionic compound is given by three particles that pull in one other. In an ionic compound, the quantity of electrons that are lost is equivalent to the quantity of electrons they picked up.
