Question
Chemistry Question on coordination compounds
Give reason for the statement. ??Ni(CN)4]2− is diamagnetic while [NiCl4]2− is paramagnetic in nature.?
In [NiCl4]2−, no unpaired electrons are present while in [Ni(CN)4]2− two unpaired electrons are present
In [Ni(CN)4]2−, no unpaired electrons are present while in [NiCl4]2− two unpaired electrons are present
[NiCl4]2− shows dsp2 hybridisation hence it is paramagnetic
[Ni(CN)4]2− shows sp3 hybridisation hence it is diamagnetic
In [Ni(CN)4]2−, no unpaired electrons are present while in [NiCl4]2− two unpaired electrons are present
Solution
In [Ni(CN)4]2− there is no unpaired electrons because CN− is a strong field ligand. Therefore it is diamagnetic in nature. 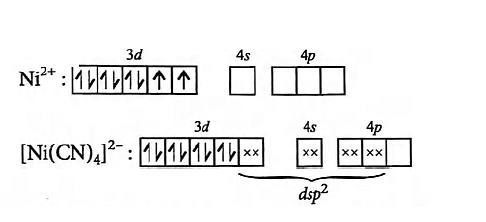 In [NiCl4]2−, there are two unpaired electrons because Cl− is a weak field ligand. Therefore, it is paramagnetic in nature.
In [NiCl4]2−, there are two unpaired electrons because Cl− is a weak field ligand. Therefore, it is paramagnetic in nature. 
