Question
Question: Give reason for the following: (A) The boiling point of ethyl alcohol is much higher than that of ...
Give reason for the following:
(A) The boiling point of ethyl alcohol is much higher than that of diethyl ether.
(B) BCl3 And BF3 are non-polar.
Solution
Hint : Boiling point of the compound depends on the nature of the bonds that molecule shares with other molecules in the compound. So to determine the boiling point of the substance we accurately determine the type of bonding present in the compound.
Complete Step By Step Answer:
Boiling point of the substance is the temperature at which the liquid form of the substance converts to its gaseous phase.
(A) So, proceeding with the question that the boiling point of ethyl alcohol is greater than the diethyl ether – the reason behind this is that though both the ethyl alcohol and the diethyl ether have the same molecular weight, but we have to keep in mind the type of bonding present in these both compounds.
Thus, in ethyl alcohol, the hydrogen of the O−H group forms intermolecular hydrogen bonding with the OH group in the other molecule. Whereas on the other hand, there is no hydrogen bonding in the diethyl ether molecule. So because of the presence of intermolecular hydrogen bonding in the ethyl alcohol it has a higher boiling point than diethyl ether.
(B) BCl3 And BF3 are non-polar – these both molecules are nonpolar because of their symmetrical original planar symmetry.
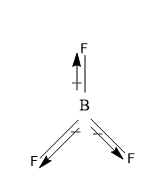
The total dipole moment of the molecule comes to be zero (μ=0) , as the resultant of the two bonds is equal in magnitude but opposite in direction to the third bond, and that’s why the total dipole moment of the molecule is zero. And thus both the BCl3 and BF3 are non-polar molecules.
Note :
The bond dipole moment uses the electric field concept to determine the polarity of the chemical bonds within the molecule. So the dipole moment is the calculation of the net molecular polarity at either end of the molecular dipole.
