Question
Question: Give reason \(Be{{F}_{2}}\) and \(B{{F}_{3}}\) both are stable, yet each has incomplete octet. Why?...
Give reason BeF2 and BF3 both are stable, yet each has incomplete octet. Why?
Solution
Electronic configuration shows the electrons that are distributed in a molecular or atomic orbital. A configuration that has the lowest electronic energy, it is known as ground state and the other configuration is excited state. A compound is said to be stable if it consists of 8 electrons in the outermost shell.
Complete step by step answer:
In BeF2 and BF3 , fluorine has lone pair of electrons, which are donated to the empty orbitals of beryllium and boron with the help of back bonding. Back bonding helps in stabilizing these compounds and hence, they exist even violating the octet rule.
1. (BF3)
As we can see, the atomic number of boron is 5
The atomic number of an element is the number of protons in its atom.
The electronic configuration of boron is 1s22s22p1 .
we can see, the number of valence electrons in boron is 3
as we can see, atomic number of fluorine is 9
The electronic configuration of fluorine is 1s22s22p5
we can see, the valence shell electron in fluorine is 7
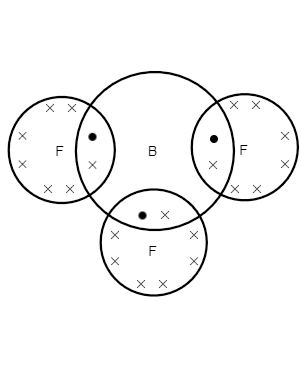
Here, we can see that the three valence electrons of boron are bonded with one out of seven valence electrons of each fluorine atom.
BF3 has six electrons for bonding, and therefore, it is electronic deficient.
2. (BeF2)
As we can see, the atomic number of beryllium is 4
The electronic configuration of beryllium is 1s22s2 .
we can see, the number of valence electrons in beryllium is 2
as we can see, atomic number of fluorine is 9
The electronic configuration of fluorine is 1s22s22p5
we can see, the valence shell electron in fluorine is 7
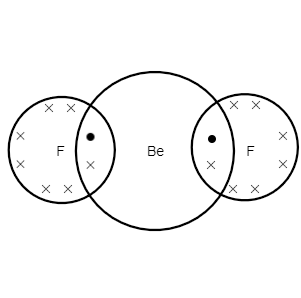
In this, two valence electrons of beryllium are bonded with one out of seven valence electrons of each fluorine atom.
BeF2 has four electrons for bonding, and therefore, it is electronic deficient.
Note: Octet rule is defined as the rule for the main group elements that tend to bond in such a way that each atom contains eight electrons in their valence shell, forming electronic configuration as a noble gas. We can calculate valence electrons using Lewis dot structure.Octet rule is only applicable for main group elements.When an atom consists of less number of electrons and requires electrons for its stability, it is known as electron deficient.
