Question
Question: Give reason: Aniline is a weaker base than ammonia....
Give reason: Aniline is a weaker base than ammonia.
Solution
Aniline is an organic compound with chemical formula C6H5NH2 . It basically consists of a phenyl group attached to an amino acid. It is the simplest aromatic aniline whereas ammonia is a compound of nitrogen and hydrogen. It is a colorless gas with chemical formula NH3 .
Complete step by step answer:
Basically, aniline is considered as the simplest aromatic amine. The chemistry of aniline is rich because the compound has been cheaply available for many years. It was first isolated in the year whereas ammonia is colorless with a pungent smell. It is lighter than air and is easily liquefied due to strong hydrogen bonding between the molecules.
Now, aniline is considered as a weaker base than ammonia. This is due to the fact that the lone pair in aniline are involved in resonance with the benzene ring and hence are not available for donation to that extent as in NH3.Moreover, according to the Lewis theory, the species which easily lose a pair of electrons are known as Lewis base. Since ammonia can easily lose electron pairs and aniline cannot, thus ammonia is considered as a stronger base than aniline. The resonating structures are as shown:
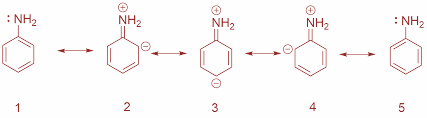
We can also say that in aniline the lone pair of nitrogen atoms is in conjugation with the πelectrons of the benzene ring and thus takes part in resonance. Hence, this pair is not available for donation while in case of ammonia it is available and thus aniline is less basic than ammonia.
Note: Liquid ammonia is the best known non-aqueous ionizing solvent. The most notable property of this compound is its ability to dissolve alkali metals to form colored, electrically conductive solutions that contain solved electrons.
