Question
Question: Give one chemical test to distinguish between the following pairs of compounds:- (1) Methylamine a...
Give one chemical test to distinguish between the following pairs of compounds:-
(1) Methylamine and dimethylamine
(2) Secondary and tertiary amines
(3) Ethylamine and aniline
(4) Aniline and benzaldehyde
(5) Aniline and N-methylaniline
Solution
Hint- To distinguish between the two compounds, we have to perform some chemical tests which are dedicated to know the nature of that compound in reaction with some other specific chemical compound.
Complete step by step solution:
(1) Methylamine and dimethylamine
To distinguish between Methylamine and dimethylamine we have to perform the following test:
- Name of the test: Carbylamine test also known as Hoffman's isocyanide test.
- This is to detect the primary amines.
CH3NH2+3KOH+CHCl3→CH3NC+3KCl+3H2O
- On heating the methylamine with the KOH alcohol solution and chloroform forms isocyanide which can be detected by its fouling smell. While the dimethylamine will not give this product that mean dimethylamine will not respond to the carbylamine test.
(2) Secondary and tertiary amines
- Name of test: Libermann Nitroso amine test.
- Secondary amines will give Libermann Nitroso Amine test in which following reaction will take place:
(CH3)2NH+HONO→(CH3)2N−N=O+H2O
- Secondary amines react with nitrous acid to form N-nitroso amines while tertiary amines will not give this test.
(3) Ethylamine and aniline
- Name of the test: Azo dye test.
Step.1-
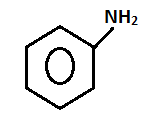 + HONO273 - 278K
+ HONO273 - 278K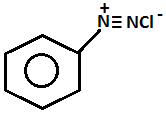 + 2H2O
+ 2H2O
Step.2-
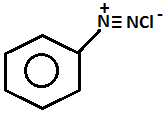 +
+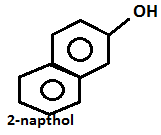 dil.NaOHpH9 - 10
dil.NaOHpH9 - 10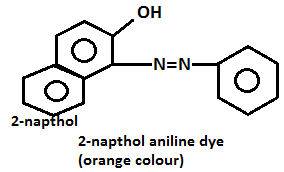 + HCl
+ HCl
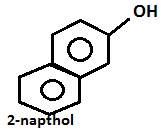
- Aniline on diazotization that means on reacting with ice cold nitrous acid solution and then coupling with 2-naphthol in alkaline solution forms brilliant orange or red dye while ethylamine will not form such dye but it will give brisk effervescence which is due to the liberation of nitrogen gas but solution will be seen clear.
(4) Aniline and benzaldehyde
- Name of the test: Azo dye test.
Step.1-
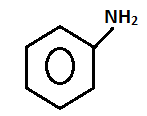 + HONO273 - 278K
+ HONO273 - 278K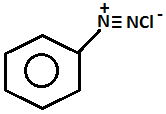 + 2H2O
+ 2H2O
Step.2-
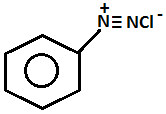 +
+ dil.NaOHpH9 - 10
dil.NaOHpH9 - 10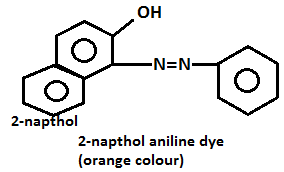 + HCl
+ HCl
- Aniline will give the azo dye test. Aniline will be reacting with ice cold nitrous acid that is by doing diazotization and coupling with the 2-naphthol in an alkaline solution will give a red dye.
- While the benzaldehyde will not give such a test.
(5) Aniline and N-methylaniline
- Name of the test: Carbylamine test.
- This is the same test performed for the distinguishing between Methylamine and dimethylamine.
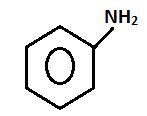
So, aniline will be performed on this test as follows:
 CHCl3KOH
CHCl3KOH 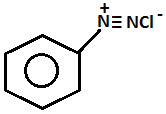
- On heating with the alcohol KOHsolution and chloroform the aniline will form methyl isocyanide which can be detected by its fouling smell.
- While N-methylaniline will not give this test.
Note- Hence we have observed that carbylamine tests can distinguish between methylamine and dimethylamine and can also distinguish between Aniline and N-methylaniline both.
We observed that azo dye test also can distinguish between two pairs of compounds that is Ethylamine and aniline and another pair of compounds Aniline and benzaldehyde.
