Question
Question: Give IUPAC name of the following compounds: (a) \({{C}}{{{H}}_3}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{OH}}...
Give IUPAC name of the following compounds:
(a) CH3CH2CH2OH
(b) HCOOH
(c) CH3CH=CHCH3
Solution
Organic chemical compounds are named based on IUPAC nomenclature. The International Union of Pure and Applied Chemistry is abbreviated as IUPAC. First we have to find the functional group present in each compound followed by the longest carbon chain or the parent chain.
Complete step by step answer:
Let’s focus on the given options.
(a) The structure of CH3CH2CH2OH is given below:
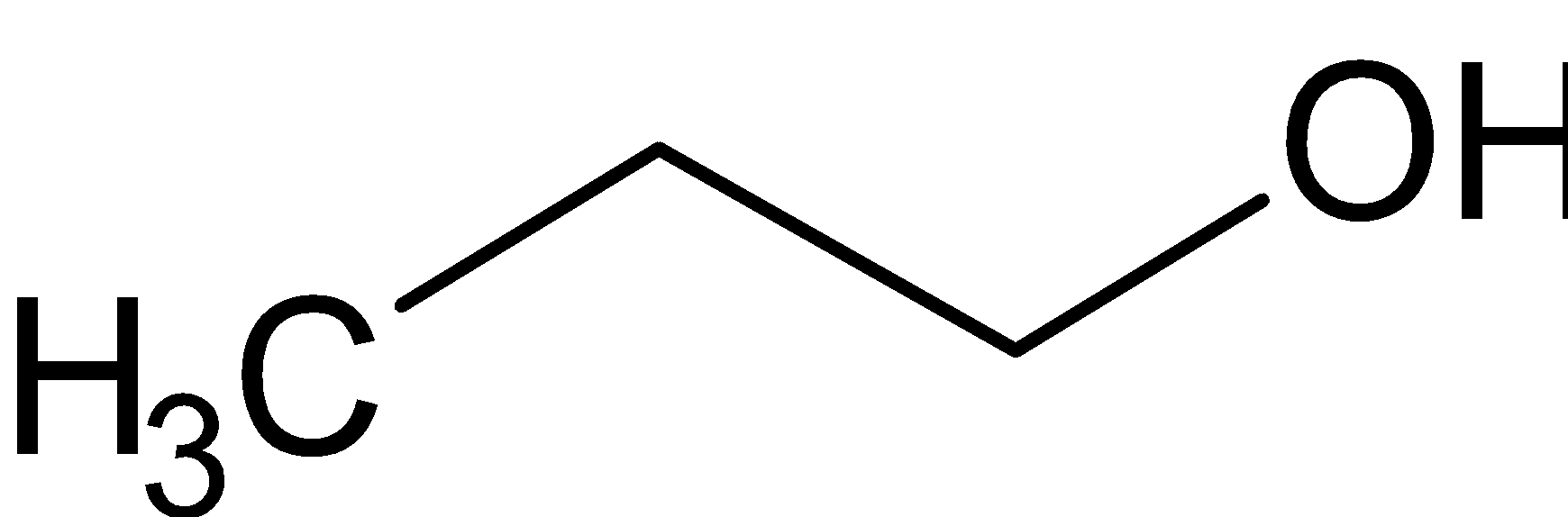
The molecular formula of this compound is C3H7O. There are three carbon atoms, seven hydrogen atoms and one oxygen atom. The functional group present in the molecule is the hydroxyl group. This is used to denote the alcohols. Thus a suffix of “ol” is used in the chemical name. “Prop” is used to denote the carbon number three. There is only a single bond. So “ane” is used. Hydroxyl group is placed at the first carbon when we count the number of carbon atoms from right to left. By combining these information, we get the IUPAC name as propan-1-ol.
(b) The structure of HCOOH is given below:
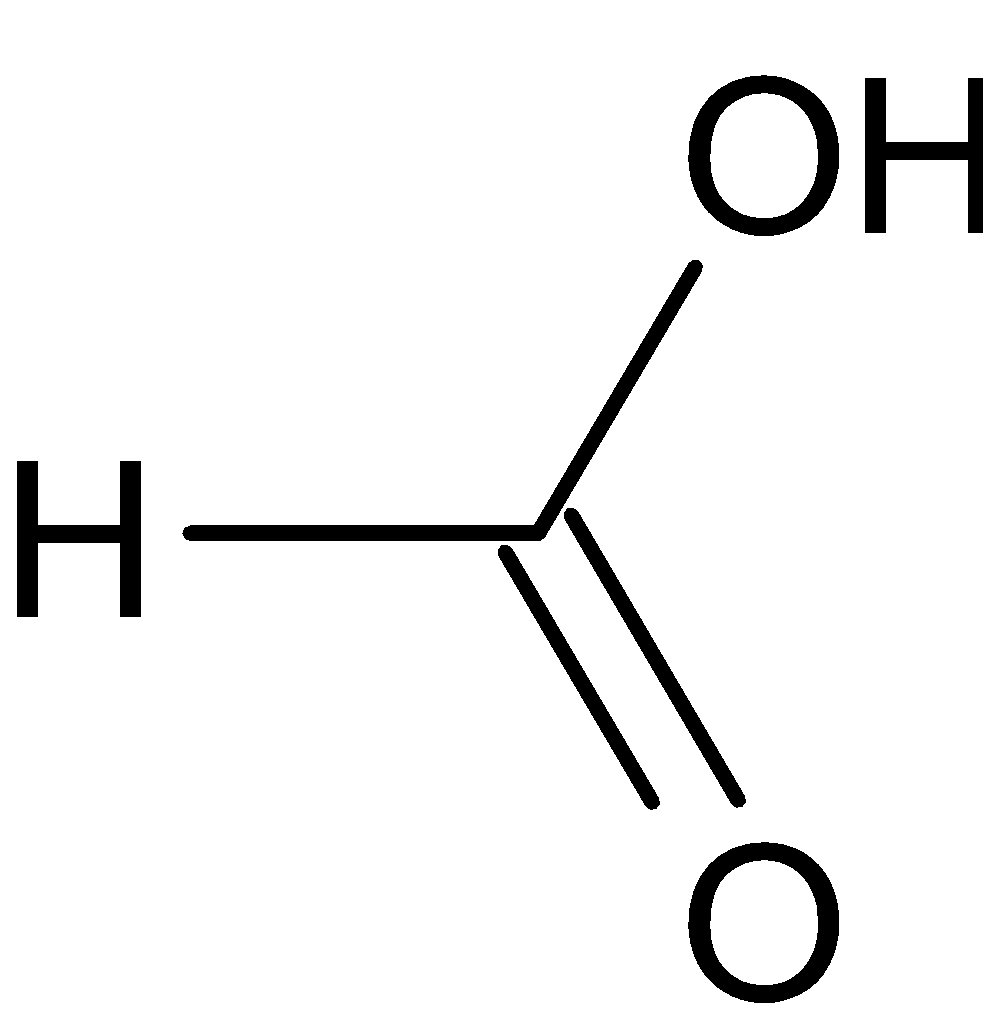
This compound has a carbonyl carbon and a hydroxyl group. The functional group present in this molecule is carboxylic group. The molecular formula of this compound is CH2O2. “Meth” is used as a parent name since the compound has only one carbon atom. Carboxylic acid functional group is denoted with “oic acid” as a suffix in the IUPAC name. This compound also has a single bond, hence “ane” is used. So the IUPAC name is methanoic acid.
(c) The structure of CH3CH=CHCH3 is given below:
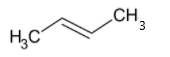
This compound is an example of alkene since it has a double bond between two carbon atoms. The molecular formula of this compound is C4H8. The double bond is placed between the second and third carbon atoms. For alkenes, “ene” is used as a suffix. “But” is used as a parent name since it contains four carbon atoms. So the IUPAC name is but-2-ene.
Note: When the substituent groups are more than one, then it must be named according to their alphabetical order. Moreover, when a substituent group has appeared two or more times, then it is named as di, tri and so on.
