Question
Question: Give examples of allylic halides....
Give examples of allylic halides.
Solution
Halides is the term used to indicate halogens when they are attached to the carbon chain. The allylic carbon atom is the carbon atom which is sp3 hybridized and it is attached to a carbon atom in the chain which is sp2 hybridized or has a double bond.
Complete answer:
There are many types of carbon chains in organic chemistry like straight-chain, branched-chain, allylic carbon chain, vinylic carbon chain, etc. When the carbon atoms have a double bond or a triple bond it is named differently.
So, we are given allylic halides, halides are the term used to indicate the halogens when they are attached to the carbon chain. The allylic carbon atom is the carbon atom which is sp3 hybridized and it is attached to a carbon atom in the chain which is sp2 hybridized or has a double bond. Halides include fluorine (F), chlorine (Cl), bromine (Br), and iodine (I).
Allylic chain means, in the chain, the last carbon atom is attached to the carbon atom having a double bond and when the last carbon atom has one substituent halogen, then it is an allylic halide.
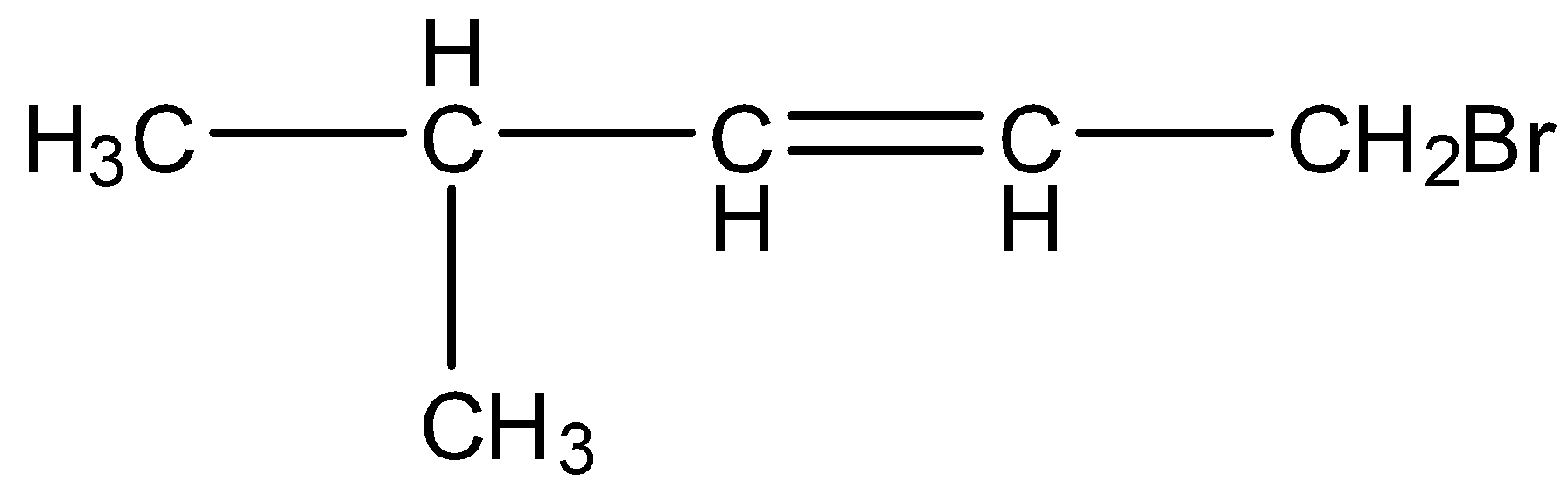
Examples of allylic halides are:
CH3−CH=CH−CH2Br
In this, the carbon atom having the bromine atom is attached with a sp2 hybridized carbon atom. Other examples are:
CH3−CH2−CH2−CH=CH−CH2Cl
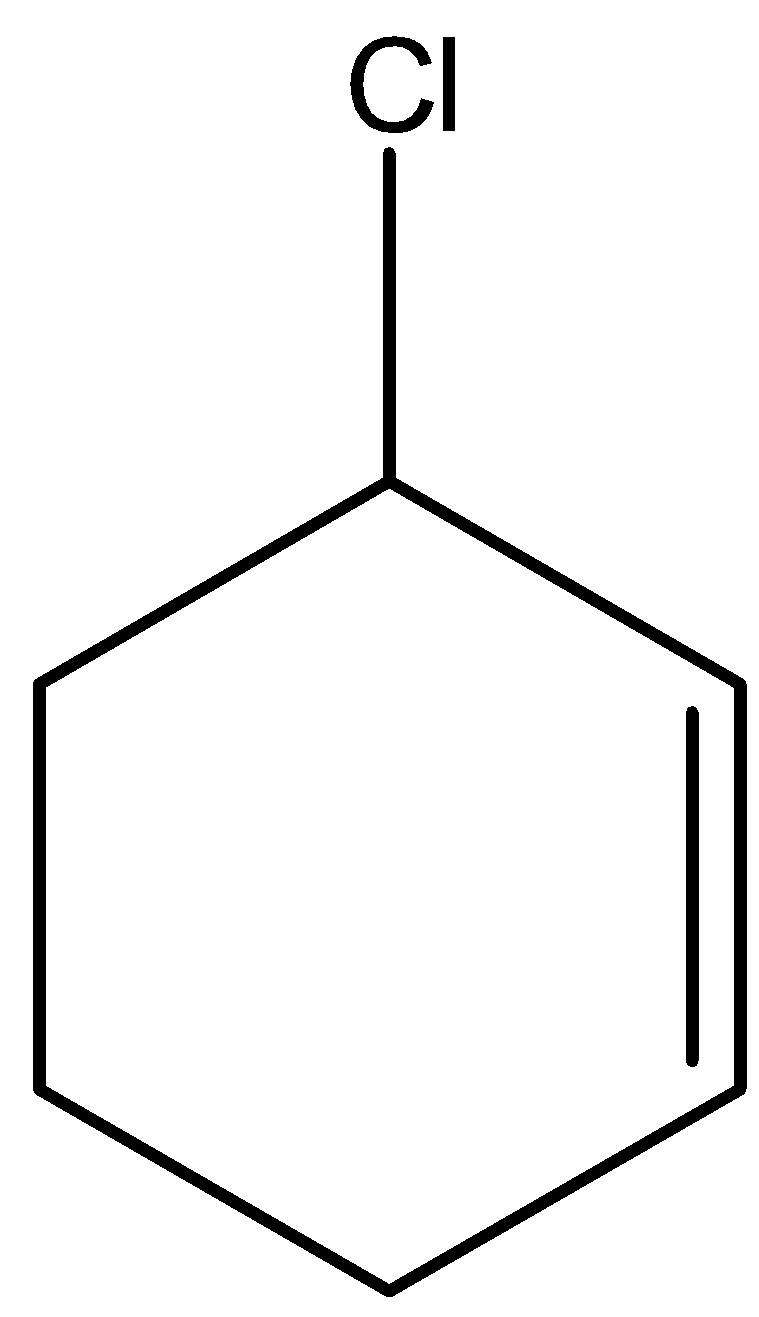
In cyclic form also we can write the allylic halide. An example of cyclic allylic halide is 2-chloro cyclohexene. Its structure is given below:
Note:
If in the chain the halogen atom is attached with the carbon atom having the double bond then it is classified as vinylic halides. An example of vinyl halide is chloroethene having formula CH2=CHCl.
