Question
Question: Give equations of the following reactions: \( A) \) Oxidation of propan \( - 1 - \) ol with alkal...
Give equations of the following reactions:
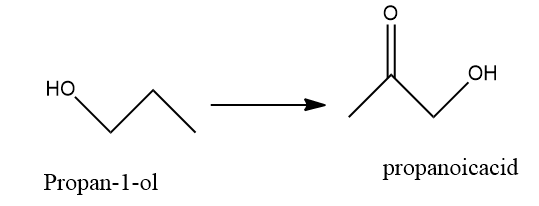
A) Oxidation of propan −1− ol with alkaline KMnO4 solution.
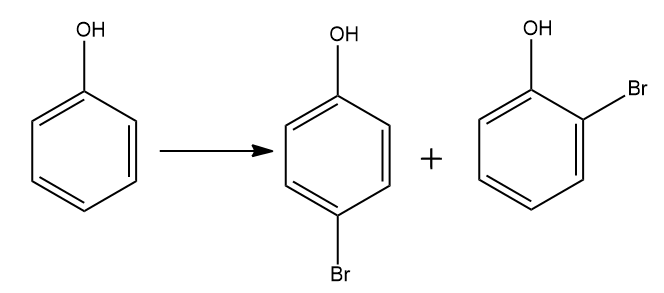
B) Bromine in CS2 with phenol.
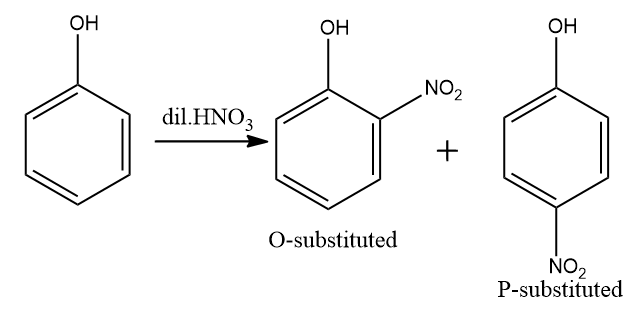
C) Dilute HNO3 with phenol.
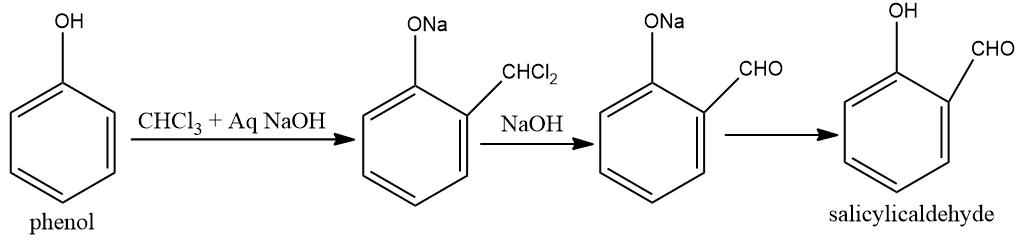
D) Treating phenol with chloroform in presence of aqueous NaOH.
Solution
To find the products of the given reactions. We must know the type of reaction which is taking place. The type of reaction which is taking place is electrophilic substitution reaction, nitration, reimer-tiemann reaction.
Complete answer:
A) Oxidation of propan- 1 -ol with alkaline KMnO4 solution: we know that the KMnO4 is a strong oxidizing agent.so, it will oxidise propan- 1 -ol to propanoic acid.
B) Bromine in CS2 with phenol.
This reaction is an electrophilic substitution reaction. In an electrophilic replacement reaction, a couple of π-bonded electrons first attack an electrophile - generally a carbocation species - and a proton is then disconnected from a neighboring carbon to restore the double bond, either in the first position or with isomerization. In this reaction two products will form first is o− substituted and p− substituted.
C) Dilute HNO3 with phenol.
This is a nitration reaction.Nitro group into a natural compound. The term additionally is applied mistakenly to the diverse cycle of shaping nitrate esters among alcohols and nitric acid. The distinction between the subsequent subatomic designs of nitro mixtures and nitrates is that the nitrogen molecule in nitro compounds is straightforwardly attached to a non-oxygen particle, while in nitrate esters, the nitrogen is clung to an oxygen particle that thusly as a rule is clung to a carbon molecule. IN this the product will form first is o− substituted and p− substituted.
D) Treating phenol with chloroform in presence of aqueous HNO3 .
This reaction is reimer- tiemann reaction.At the point when phenols for example phenol is treated with chloroform within the sight of sodium hydroxide, an aldehyde bunch is presented at the ortho position of benzene ring prompting the arrangement of o -hydroxybenzaldehyde. The response is famously known as the Reimer Tiemann reaction.
Note:
Remember when the reaction takes place two products are formed, the major and the minor product. The major product is "more suited" than the minor product.
