Question
Question: Give Arrhenius an equation. How will you determine the activation energy of a reaction by graph meth...
Give Arrhenius an equation. How will you determine the activation energy of a reaction by graph method?
Solution
Arrhenius equation is useful for calculating the energy of activation of a reaction having rate constant “k” at temperature “T”
Activation energy is the excess energy that the reacting molecules whose energy is less than threshold energy must acquire to react to give the products.
Activation energy can be obtained graphically by the slope of lnK vs T1
Complete step by step answer:
Arrhenius equation for calculating the energy of activation of a reaction having rate constant “k” at temperature “T” can be written as follows,
k=AeRT−Ea......(1)
Where,
k- Rate constant
A- Frequency factor
Ea- Arrhenius activation energy
R-Gas constant
T-Temperature
Taking the natural logarithm of equation (1) on both sides,
(1)⇒lnk=lnA+(RT−Ea)
⇒lnk=(RT−Ea)+lnA.......(2)
Equation (2) depicts the y=mx+c equation where
y=lnk
m=R−Ea
x=T1
c=lnA
Thus, the plot lnKvsT1 can be drawn as,
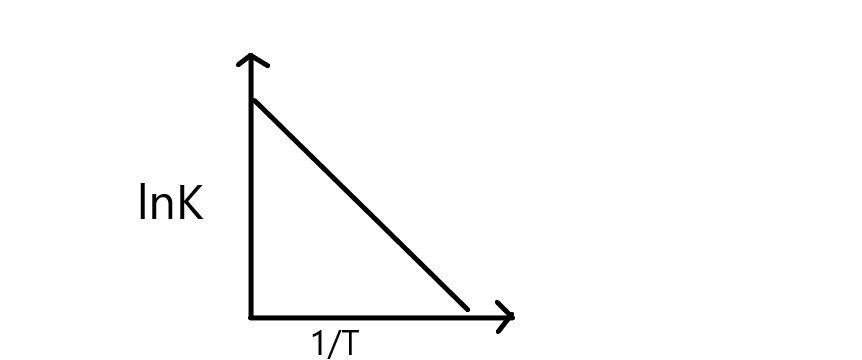
From the graph, it depicts that rate constant decreases with increase in T1 resulting in a straight line, and its slope can be equal to R−Ea
Thus, from the slope lnKvsT1, we can calculate the activation energy of a reaction.
Note: The slope of the natural logarithm of the Arrhenius equation is negative. m=R−Ea Where R=8.314JK−1mol−1
The rate constant is affected by temperature whereas activation energy is independent of temperature. The activation energy is affected by the use of catalysts. . Generally, activation energy is the excess energy required by the molecules to react to yield a product. When a catalyst is used, the activation energy barrier gets decreased and it makes the reaction happen faster.
When the temperature increases, more molecules are undergoing collision and temperature cannot change the activation barrier of a reaction.
