Question
Question: Give an example of a compound which can form linkage isomers?...
Give an example of a compound which can form linkage isomers?
Solution
Hint : Isomers are compounds having the same molecular formula but the arrangement of atoms in space is different. And they differ in properties also. The isomers show phenomena known as isomerism in which the compounds with the same molecular formula show different structures. There are different types of isomerism mainly structural and stereoisomerism which are furthermore classified into few.
Complete Step By Step Answer:
Coordination compounds also show isomerism. One of which is linkage isomerism and the compound which shows linkage isomerism is called linkage isomers.
Linkage isomerism arises in coordinating compounds containing ambidentate ligands. These ligands are capable of coordinating in more than one way with the central metal atom. This usually occurs when the ligand has two alternate donor atoms. The best known ligands showing this isomerism are: SCN− / NCS− and NO2−/ ONO−.
Let us understand it by taking an example [CO(NH3)5(SCN)]2+ and [CO(NH3)5(NCS)]2+
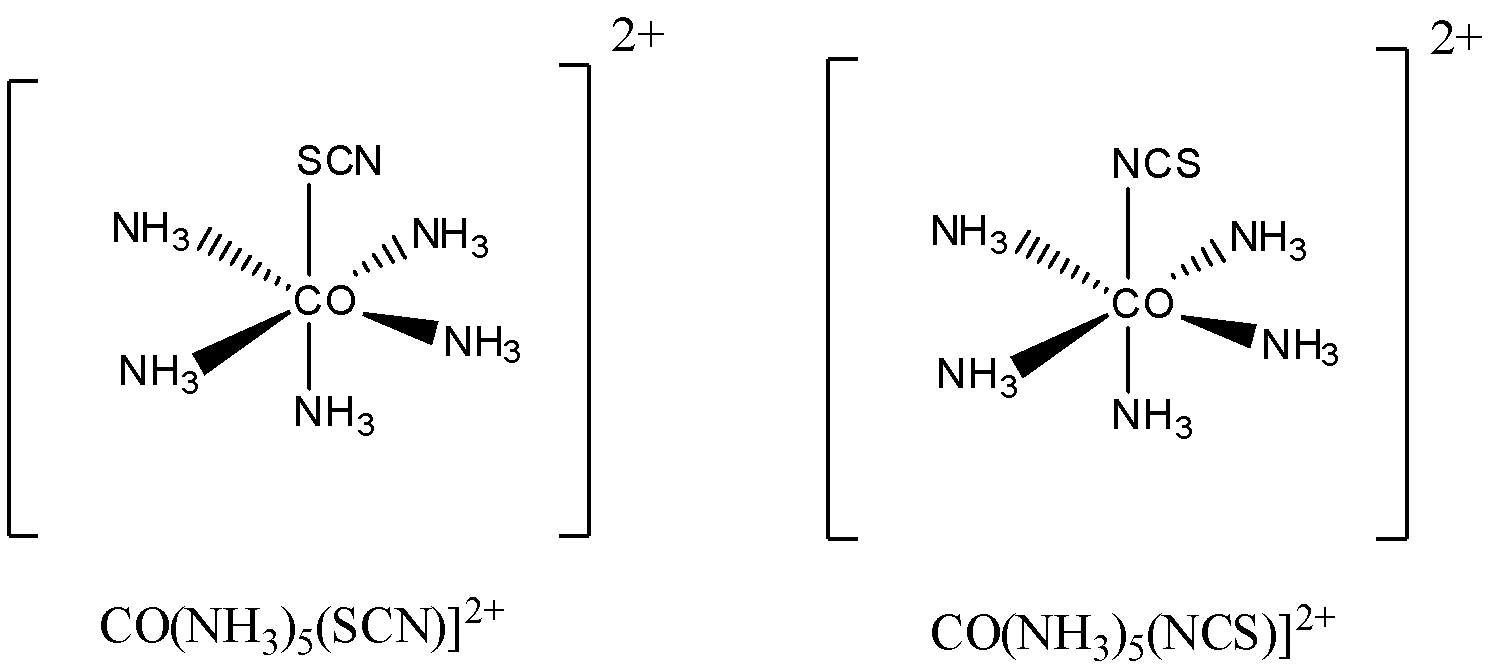
It is clearly visible in the above example how SCN− / NCS− are coordinating with the same central metal atom but two different donor atoms S and N .
Here a single ligand is coordinating in different ways (with different donor atoms) but the molecular formula remains the same as we are not changing the ligand only change will occur in writing their names.
We can take another example with NO2−/ ONO− as ligands.
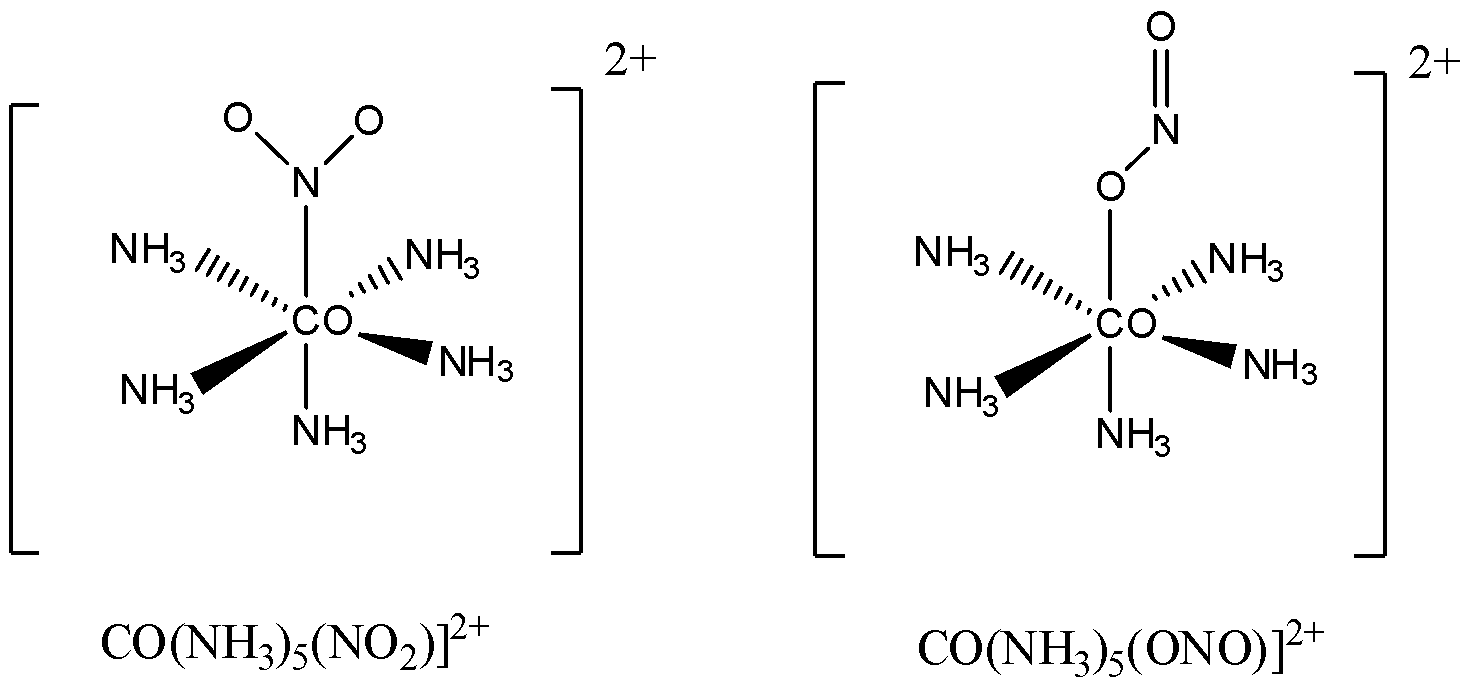
In this example also it can be seen that NO2 is coordinating with the central atom in two different ways.
Therefore the example of compound showing linkage isomer is [CO(NH3)5(SCN)]2+ , [CO(NH3)5(NCS)]2+ and [CO(NH3)5(NO2)]2+ , [CO(NH3)5(ONO)]2+
Note :
Linkage isomers are only those compounds which can coordinate with a central atom with two or more donor atoms. These ligands are called ambidentate. It can be only shown by coordination compounds and not normal compounds.
