Question
Question: Give all possible isomers of alkane with formula \({C_4}{H_{10}}\)....
Give all possible isomers of alkane with formula C4H10.
Solution
Hint: Isomers are compounds that have the same molecular formula but different structural formula. They can also be said to be compounds with different connectivity of atoms in molecules. To determine isomers, we have to count to count the number of each atom in each molecule. In an isomer the molecular formula will be the same for both the molecules but the arrangement of atoms will be different.
Complete answer:
Butane is an alkane. It has four carbon atoms and its molecular formula is C4H10.
It has two isomers and they are: -
a. n-butane and
b. isobutane.
Now we will be going through the structure of n-butane and isobutane.
Both of these molecules have the same molecular formula that is C4H10. Carbon atoms are in straight-line in n-butane.
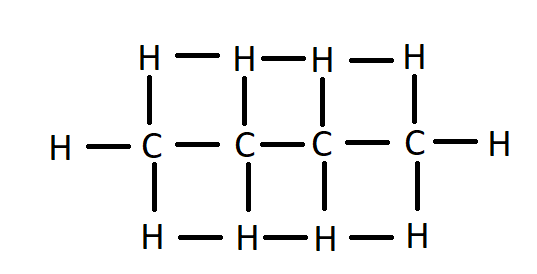
Structure of n-butane
In isobutane, there is a side chain carbon atom in the molecule.
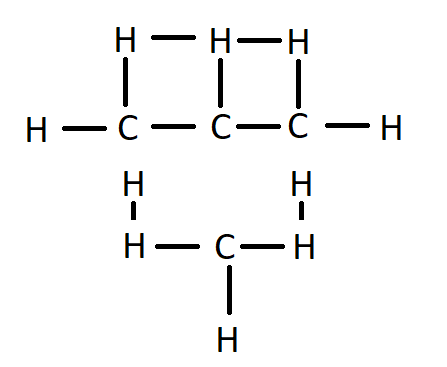
Structure of isobutane
So, there are different connectivity of atoms and thus they are isomers of each other.
Note: For such types of questions we must remember the property and definition of isomer. Here arrangement of atoms determines the name of the compounds.
