Question
Question: Give a test to differentiate \({ 1,1-dichloroethane }\) and \({ 1,2-dichloroethane }\): A) \({ 2,4...
Give a test to differentiate 1,1−dichloroethane and 1,2−dichloroethane:
A) 2,4−DNP and then aq. KOH
B) aq. KOH and then 2,4−DNP
C) NaHSO3
D) Lucas reagent
Solution
The only difference between 1,1-dichloroethane and 1,2-dichloroethane is that the two chlorine atoms are present on the same carbon atom in case of 1,1-dichloroethane, they are present in the adjacent carbon atoms in case of 1,2-dichloroethane. So we can oxidize both the compounds and 1,1-dichloroethane will form acetaldehyde while 1,2-dichloroethane will form ethan-1,2-diol. There is a particular reagent which can be used to distinguish between these two and it gives a yellow precipitate in presence of aldehydes.
Complete answer:
Lucas reagent: It is a solution of anhydrous zinc chloride in concentrated hydrochloric acid. This solution is used to differentiate between primary, secondary, and tertiary alcohols of low molecular weight. This is a replacement wherein the chloride replaces a hydroxyl group.
NaHSO3 It is known as Sodium bisulfite is a weakly acidic compound. It is usually used as a mild reducing agent, so typically used as an aqueous solution.
The reaction of 1,1−dichloroethane and 1,2−dichloroethane with 2,4−DNP firstly, and then aqueous KOH won’t occur as 2,4−DNP is used to differentiate between an aldehyde and a ketone, so firstly when aqueous KOH reacts with these compounds then geminal diol will be formed. Hence, this is not the correct option.
When 1,1−dichloroethane reacts with aq. KOH then ethanal will be produced which is a pungent-smelling aldehyde which when further reacts with 2,4−DNP then 2,4−dinitrophenylhydrazine will be produced which is insoluble and yellow colored precipitate.
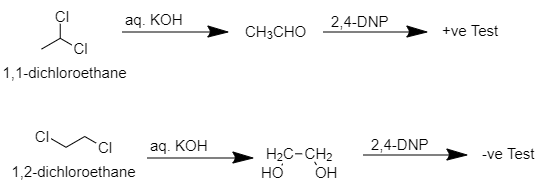
When 1,2−dichloroethane reacts with aq. KOH then ethan−1,2diol will be produced which is odorless and when it reacts with 2,4−DNP then no precipitate formation occurs.
Therefore, we can say that we can differentiate 1,1−dichloroethane and 1,2−dichloroethane from aq. KOH and then 2,4−DNP.
So, The correct option is B.
Note: The possibility to make a mistake is that you may choose option A. But to differentiate 1,1-dichloroethane and 1,2-dichloroethane we have to add aq. KOH and then 2,4-DNP will be used. Do not make a mistake by thinking that adding 2,4-DNP first and then aq. KOH will yield the same result. The 2,4-DNP gives yellow precipitate only for aldehydes. So we need to oxidize the compounds by aq. KOH first then we have to use 2,4-DNP for getting the result.
