Question
Question: Give a simple chemical test to distinguish between aniline and N, N-dimethyl aniline....
Give a simple chemical test to distinguish between aniline and N, N-dimethyl aniline.
Solution
Amines are the compounds having −NH2 group attached to the parent group. Both aniline and N,N-dimethyl aniline is included in the amine family. To differentiate between the given compounds, we have to understand the nature of both compounds in reaction with certain chemical compounds.
Complete step by step solution:
The structure of aniline and N,N-dimethyl aniline is given below:
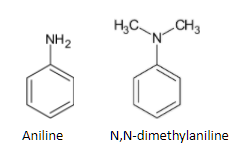
Aniline is a primary amine while N,N-dimethyl aniline is a tertiary amine. So we can use carbylamine tests to distinguish them. The Carbylamine test is also known as Hoffman’s isocyanide test. Primary amines give positive results for this test. When aniline is heated with chloroform and ethanolic potassium hydroxide, a foul smell is produced. This is because of the production of isocyanides. The test is given as a carbylamine test because isocyanides are also known as carbylamines. The reaction is given below:
C6H5NH2+3KOH+CHCl3→3CH3NC+3KCl+H2O
While N,N-dimethyl aniline, being a tertiary amine, does not react with chloroform and ethanolic potassium hydroxide.
i.e. C6H5N(CH3)2+CHCl3+3KOHΔ No reaction
Thus this compound does not undergo carbylamine tests.
Hence we can say that aniline and N,N-dimethyl aniline can be distinguished by carbylamine test.
Additional information:
Aliphatic amines and aromatic amines can be distinguished using azo dye test. This produces a dye which is orange or red. Aliphatic amines does not respond to this test. It produces nitrogen gas which causes a brisk effervescence.
Note: Carbylamine test is used to distinguish primary and tertiary amines. While secondary and tertiary amines can be distinguished with the help of Libermann’s test. It is generally called the Libermann nitroso test. This is done by reacting the amines with HONO molecule giving nitroso group.
