Question
Question: Give a brief description of the basic elementary process involved in the photoelectric emission in E...
Give a brief description of the basic elementary process involved in the photoelectric emission in Einstein’s picture. When a photosensitive material is irradiated with the light of frequency v. The maximum the speed of electrons is given byVmax. A plot ofVmax2is found to vary with frequencyvas shown in the figure. Use Einstein’s photoelectric equation to find the expression for
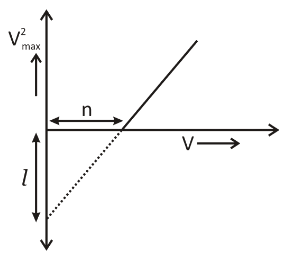
(a) Planck's constant
(b) Work function of the given photosensitive material in terms of the parameters l , n and mass m of the electron.
Solution
First of all, we will have to find the expression for the maximum kinetic energy. We know that an electron is not emitted if the frequency is less than the threshold frequency. In that case the kinetic energy is zero. There will be two velocities. We will have to manipulate accordingly and obtain the result.
Formula used:
Einstein explained the photoelectric effect on the basis of quantum theory. As we know the formula of work function, hv0=λ0hc
Here c is the speed of light, h is the Planck’s constant and ν is the frequency.
By substituting all the values in the given formula, we can find the value of Planck’s constant and the work function, W. If we define the work-function of a metal, it is the minimum energy required to eject one electron from metal.
Complete step by step solution:
(a) Letv be the frequency,hv be the energy of the proton,W be a work function and EK. Be the maximum kinetic energy of the photoelectron.
hv=W+EK
⇒EK=hv0−W …… (A)
Let v0be threshold frequency, no electron will be emitted if frequency of incident light is less than the threshold frequency.
0=hv0−W
Let λ0 be the threshold wavelength.
Then, vo=λ0c
Work function
w = \dfrac{1}{2}m\left( {\dfrac{1}{n}} \right)n \\
\therefore w = \dfrac{1}{2}ml \\
