Question
Question: Give a balanced equation to convert methyl cyanide to ethanol....
Give a balanced equation to convert methyl cyanide to ethanol.
Solution
We know that amine is a functional group whose structure is (−NH2). Here, first we have to convert the cyanide to an amine. Then amine can be converted to the required alcohol by reacting with acids.
Complete step by step answer:
Let’s first understand the cyanide functional group. Cyanide is a functional group whose structure is (C≡N). Some examples of cyanide containing compounds are methyl cyanide CH3−CN , ethyl cyanide (CH3−CH2−CN)etc.
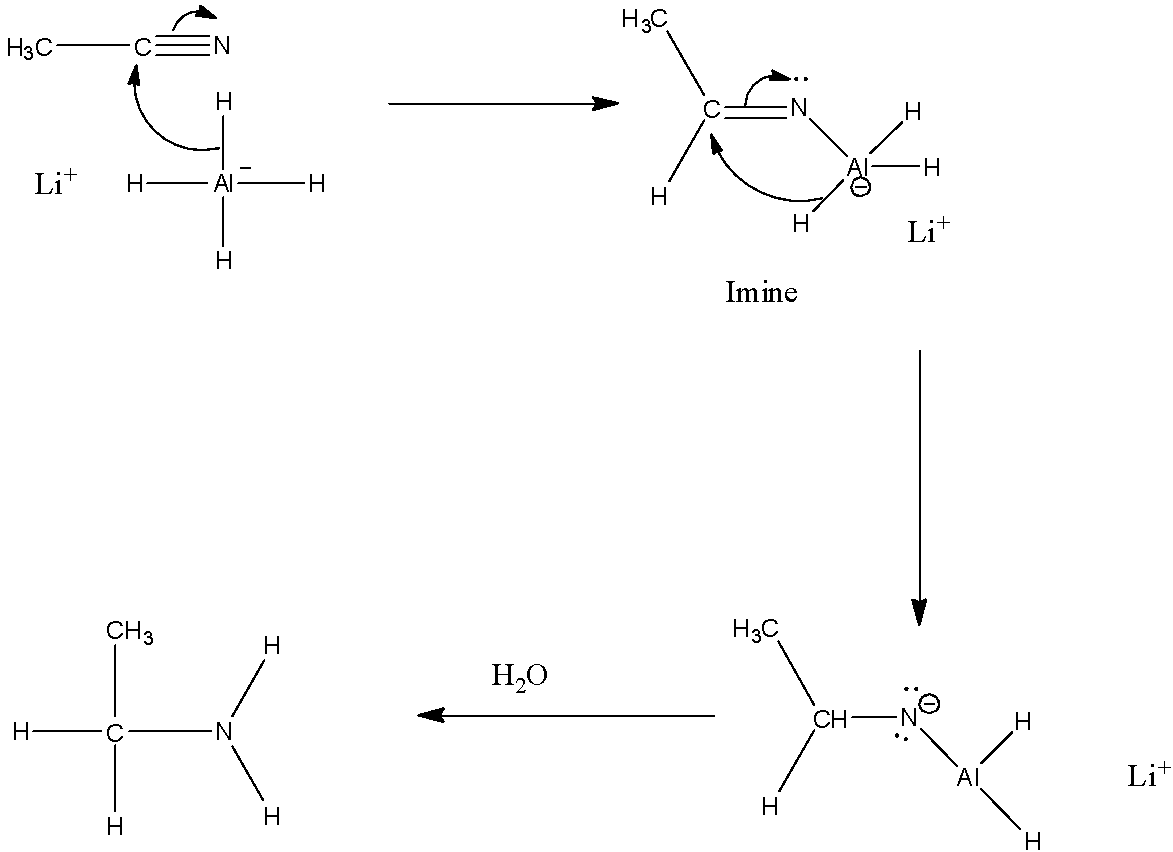
Here, we have to reduce methyl cyanide to methyl amine. This can be achieved by reacting methyl cyanide with a reducing agent like LiAlH4
.
Let’s discuss how LiAlH4 reduces methyl cyanide to ethanamine in detail. During this reaction, the nucleophilic attack of hydride ions on the electrophilic carbon of the nitrile takes place to form an imine. Then, the second nucleophilic attack of hydride takes place to form dianion. Then, the conversion of dianion to amine occurs by addition of water.
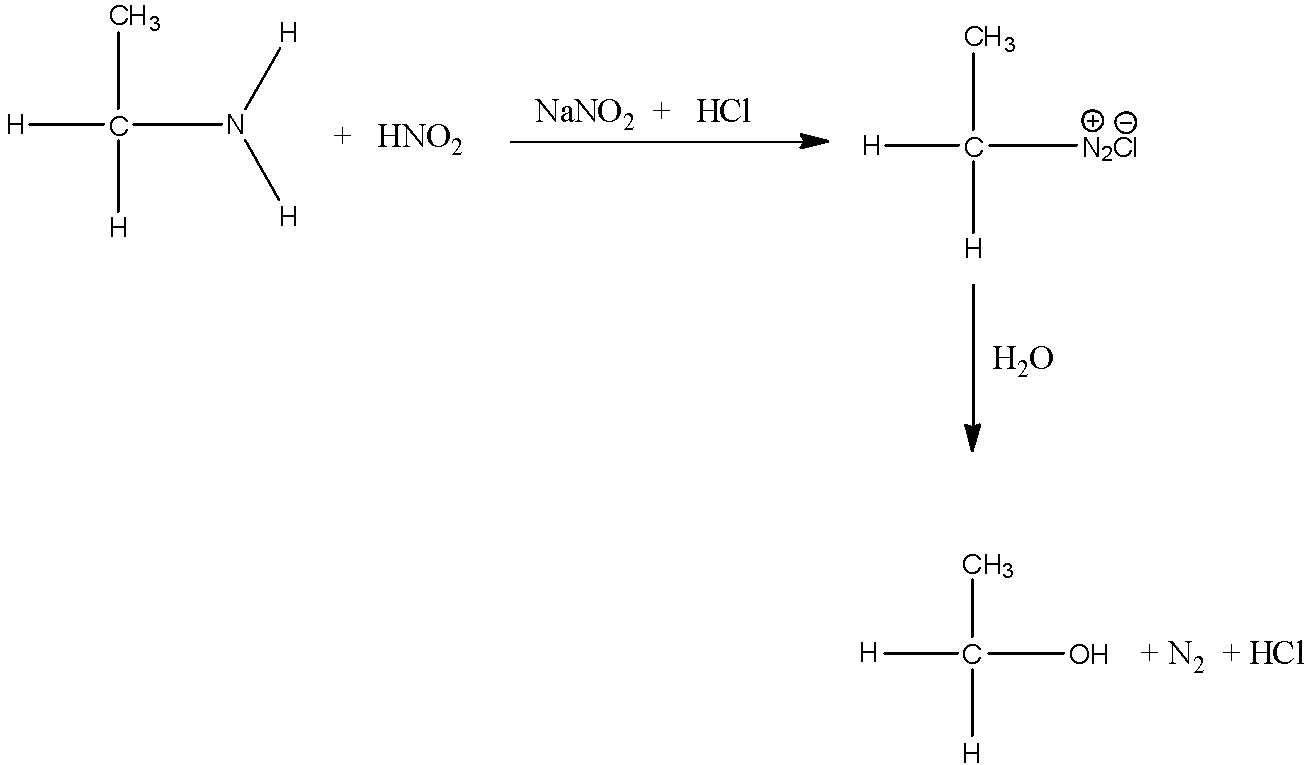
Now, we have to convert ethanamine to ethanol. Ethanol can be formed by reacting the ethanamine with nitrous acid to form aliphatic diazonium salt. Diazonium salt formed is very unstable. Hence, it liberates nitrogen gas and forms alcohol. The reaction can be shown as follows:
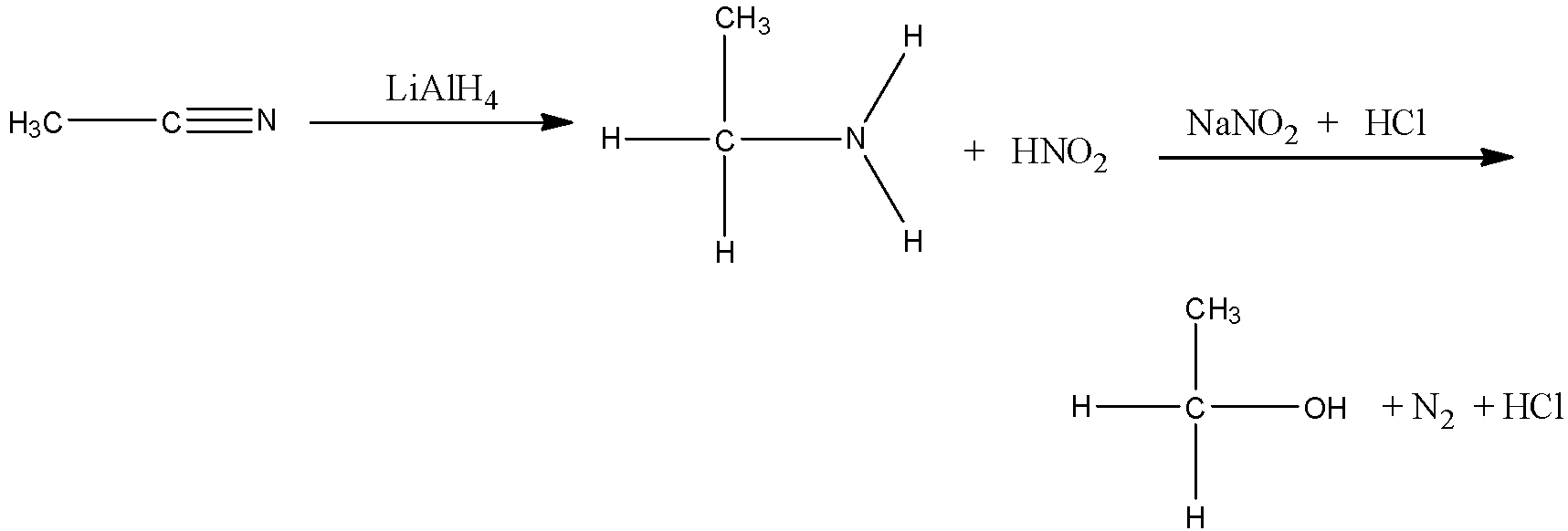
Therefore, the complete reaction of conversion of methyl cyanide to ethanol is,
Note: Remember that there are three types of amine, primary amine, secondary amine and tertiary amine.The amine in which one hydrogen atom of ammonia is replaced by an alkyl group is termed as primary amine. One example isCH3−NH2
.
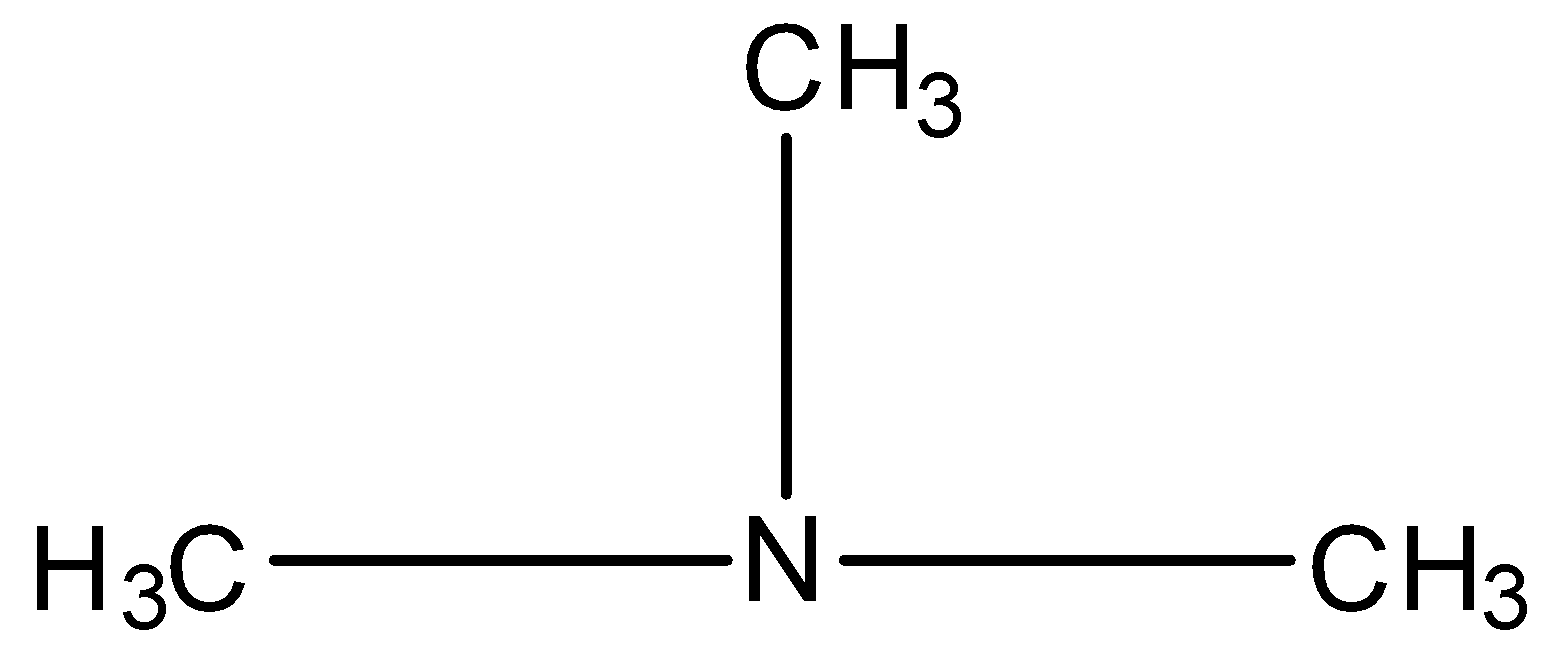
Secondary amine is the amine in which two hydrogen atoms of ammonia are replaced by two alkyl groups, such as, $$
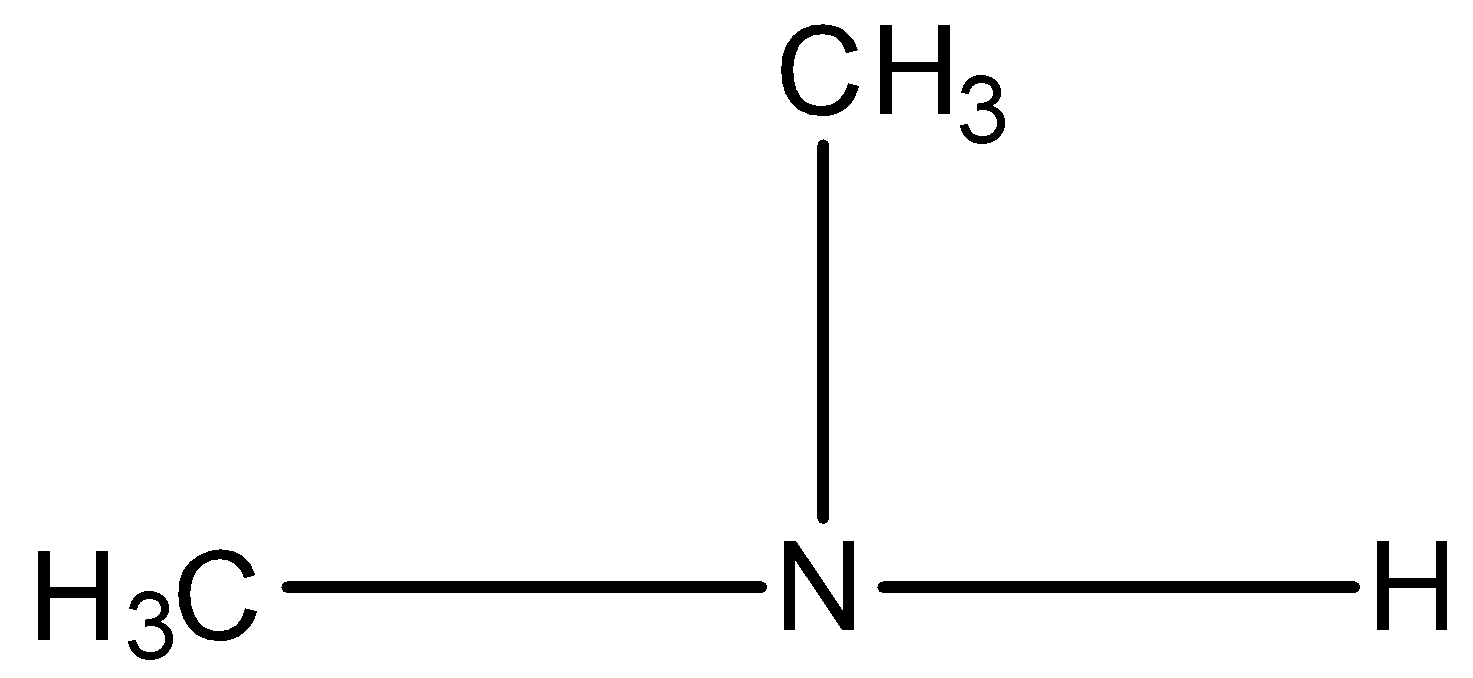
Tertiary amine is the amine in which, all three hydrogen atoms are replaced by alkyl groups, such as,