Question
Question: Geometry of central atom in \({\text{SN}}{{\text{F}}_{\text{3}}}\) is:...
Geometry of central atom in SNF3 is:
Solution
To determine the geometry we will draw the Lewis structure. Lewis structure shows the electrons around the atoms of a molecule. According to VSEPR theory, the sum of sigma electrons pairs and lone pairs present around the central atom give the geometry.
Complete answer:
Write the Lewis structure as follows:
1. Write the basic structure.
2. Write the central atom around which writes all atoms of the molecule. The least electronegative atom is the central atom.
3. Count total valence electrons.
4. Two electrons are used in the formation of a bond.
5. Count the total electron used in bond formation.
6. Subtract the electrons used in bond formation from the total valence electrons.
7. Arrange the remaining electrons around each atom to complete the octet.
Lewis's structure gives an idea of geometry.
According to valence shell electron pair repulsion theory, the electron pairs get arranged around the central to minimize the repulsion.
The geometry is decided based on the number of sigma and lone pairs around the central atom.
| Number of electron pairs | Geometry |
|---|---|
| 2 | Linear |
| 3 | Trigonal planar |
| 4 | Tetrahedral |
The Lewis structure of is as follows:
Total valence electrons are as follows:
=(6×1)+(5×1)+(7×3)
=32
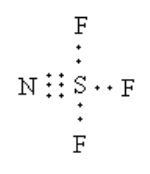
The total electrons used in the bonds are 12.
The remaining valence electrons are,
=32−12
=20
Arrange the 20 electrons to complete the octet of each atom.
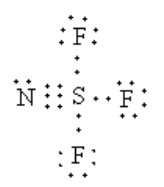
The sigma electron pairs around the sulphur atom is four. So, the geometry of sulphur is tetrahedral.
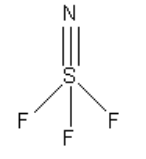
Note:
Each atom requires eight electrons to complete an octet except hydrogen. The hydrogen requires two electrons to complete the octet. The least electronegative atom in SNF3 is sulphur. Geometry is decided on the basis of sigma electron pairs and lone pairs of central atoms only. The pi bonds do not count in deciding the geometry.
