Question
Question: Which is the weakest base among the followings?...
Which is the weakest base among the followings?
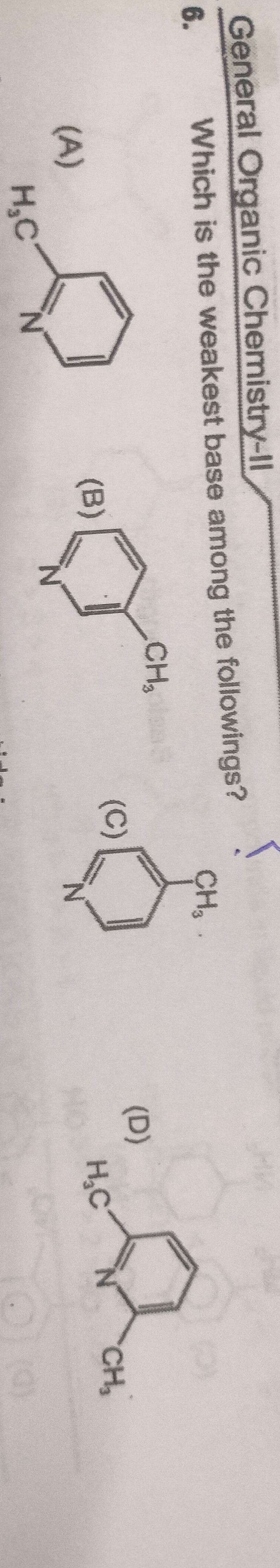
H3C N
N CH3
N CH3
H3C N CH3
A
Solution
The basicity of an amine or a heterocyclic nitrogen compound like pyridine depends on the availability of the lone pair of electrons on the nitrogen atom for donation to a proton (H⁺).
- Electron-donating groups (EDGs) increase the electron density on the nitrogen atom, making its lone pair more available and thus increasing the basicity.
- Electron-withdrawing groups (EWGs) decrease the electron density on the nitrogen atom, making its lone pair less available and thus decreasing the basicity.
In this question, all options are pyridine derivatives. The methyl group (-CH₃) is an electron-donating group primarily through its positive inductive effect (+I) and hyperconjugation.
Let's analyze each option:
-
(A) Pyridine: This is the parent compound with no substituents. Its basicity serves as a reference.
-
(B) 3-Methylpyridine (β-Picoline): It has one methyl group at the meta position (position 3). The methyl group exerts a +I effect, which is weaker at the meta position compared to ortho or para, but it still increases the electron density on the nitrogen slightly. Thus, it is a stronger base than pyridine.
-
(C) 4-Methylpyridine (γ-Picoline): It has one methyl group at the para position (position 4). The methyl group exerts a +I effect and hyperconjugation, both of which are effective in stabilizing the positive charge on the nitrogen in the conjugate acid through resonance. This significantly increases the electron density on the nitrogen, making it a stronger base than pyridine and 3-methylpyridine.
-
(D) 2,6-Dimethylpyridine (α,α'-Lutidine): It has two methyl groups at the ortho positions (positions 2 and 6). Each methyl group exerts a +I effect. The combined electron-donating effect from two methyl groups is substantial. While there might be some steric hindrance to protonation, the strong electron-donating effect typically dominates in these cases, making it a very strong base among the given options.
Comparison of Basicity:
Since methyl groups are electron-donating, all methyl-substituted pyridines (B, C, D) will be stronger bases than unsubstituted pyridine (A).
Therefore, among the given options, unsubstituted pyridine (A) will be the weakest base.
The order of basicity (from weakest to strongest) is generally:
Pyridine (A) < 3-Methylpyridine (B) < 4-Methylpyridine (C) < 2,6-Dimethylpyridine (D)
