Question
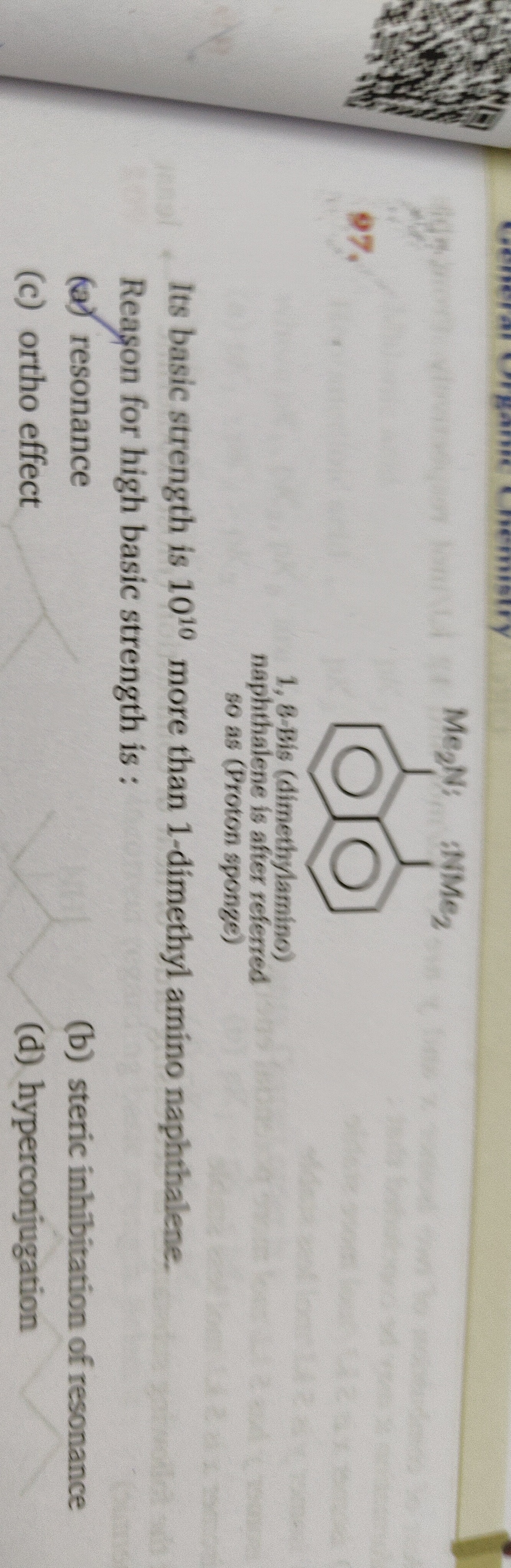
Question: Its basic strength is $10^{10}$ more than 1-dimethyl amino naphthalene. Reason for high basic stren...
Its basic strength is 1010 more than 1-dimethyl amino naphthalene.
Reason for high basic strength is:
A
resonance
B
ortho effect
C
steric inhibitation of resonance
D
hyperconjugation
Answer
ortho effect
Explanation
Solution
The high basic strength of 1,8-bis(dimethylamino)naphthalene (proton sponge) is due to steric repulsion between the peri-positioned dimethylamino groups. This forces the nitrogen lone pairs to be directed outwards, increasing their accessibility for protonation. This steric hindrance also inhibits resonance of the lone pairs with the naphthalene ring. The "ortho effect" is the general term for the influence of peri substituents, encompassing these steric effects that enhance basicity.
