Question
Question: Arrange the following (w, x, y, z) in decreasing order of their boiling points: ...
Arrange the following (w, x, y, z) in decreasing order of their boiling points:
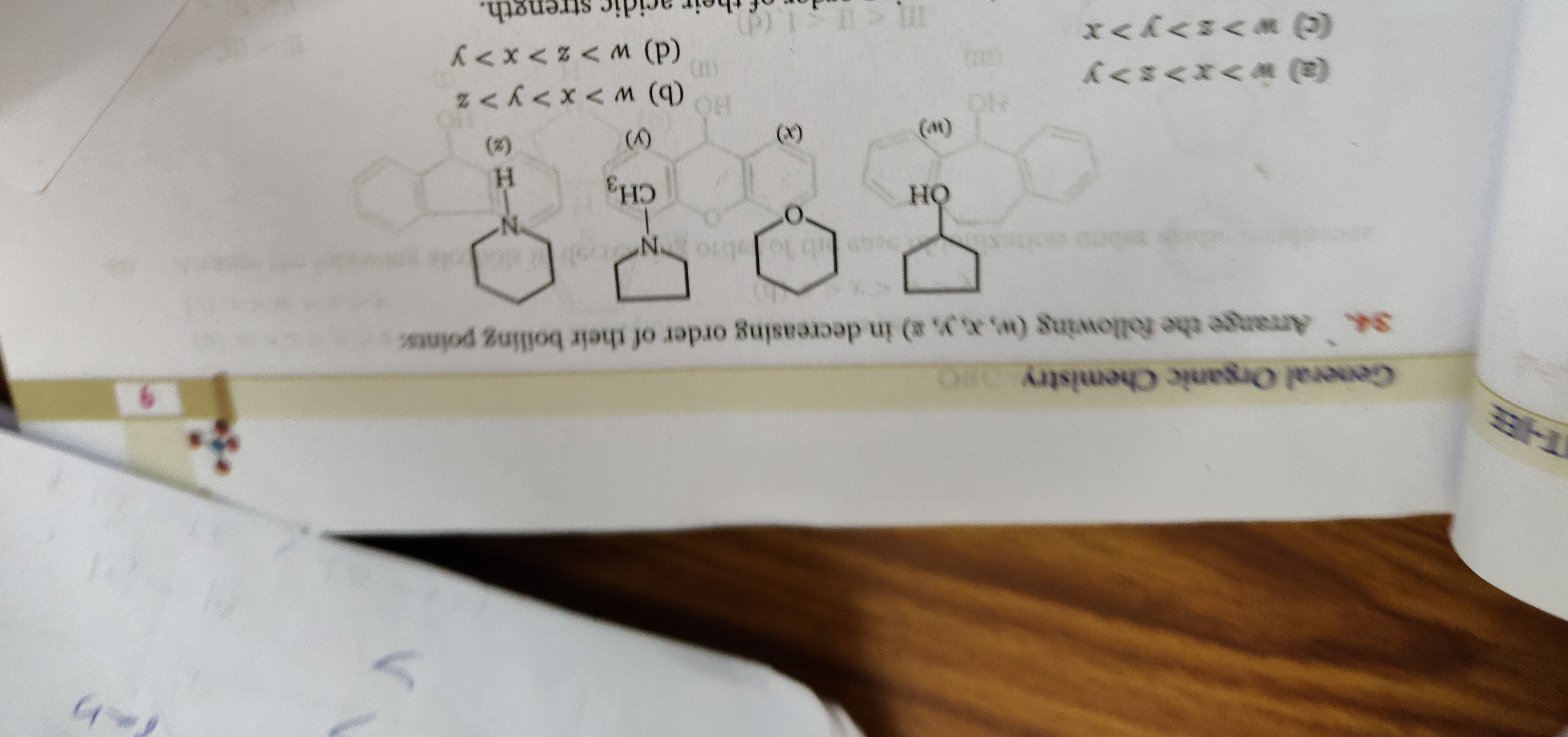
w>x>z>y
w>x>y>z
w>z>y>x
w>z>x>y
b
Solution
The boiling points of organic compounds are influenced by molecular weight and intermolecular forces, primarily van der Waals forces, dipole-dipole interactions, and hydrogen bonding.
-
Identify the structures:
- w: 1-Naphthol. Molecular formula: C10H8O. Molecular weight: 144 g/mol. Boiling point: 288 °C.
- y: 2-Naphthol. Molecular formula: C10H8O. Molecular weight: 144 g/mol. Boiling point: 285 °C.
- z: Saturated bicyclic alcohol (e.g., Decalol). Molecular formula: C10H17OH. Molecular weight: 156 g/mol. Boiling point: ~255-260 °C.
- x: Methylnaphthol. Molecular formula: C11H10O. Molecular weight: 158 g/mol. Boiling point: ~285 °C (for isomers like 1-methyl-2-naphthol or 2-methyl-1-naphthol).
-
Analyze intermolecular forces: All compounds possess a hydroxyl (-OH) group, enabling hydrogen bonding, which significantly increases boiling points. Phenols (w, y, x) generally have higher boiling points than saturated alcohols (z) of similar molecular weight due to stronger hydrogen bonding and resonance effects.
-
Compare boiling points:
- w vs. y: 1-Naphthol (w) has a slightly higher boiling point (288 °C) than 2-Naphthol (y) (285 °C). So, w > y.
- z vs. others: Saturated bicyclic alcohols like decalol (z) have lower boiling points (~255-260 °C) compared to naphthols and methylnaphthols due to the absence of resonance stabilization and potentially weaker intermolecular forces overall, despite having a slightly higher molecular weight than naphthols. Thus, z is likely the lowest.
- x vs. y: Methylnaphthols (x) have molecular weights (158 g/mol) slightly higher than naphthols (144 g/mol). Their boiling points are comparable to naphthols, often slightly higher or similar depending on the specific isomer. For example, methylnaphthols have boiling points around 285 °C. If we assume x has a boiling point slightly higher than y, e.g., BP(x) = 286 °C and BP(y) = 285 °C.
-
Establish the order:
- w (288 °C) is the highest.
- z (~255-260 °C) is the lowest.
- Comparing x and y: if BP(x) > BP(y), then the order is w > x > y > z.
-
Match with options: The order w > x > y > z fits the observed boiling points and general trends.
