Question
Question: Gem dihalide on hydrolysis gives: a.) vicinal diol b.) geminal diol c.) carbonyl compound d....
Gem dihalide on hydrolysis gives:
a.) vicinal diol
b.) geminal diol
c.) carbonyl compound
d.) carboxylic acid
Solution
Hint : Geminal dihalide hydrolysis is an organic reaction in which the gem-halide reacts with water or undergo hydrolysis in an alkaline medium. There are two products which can be formed.
Complete step by step solution : Geminal dihalides are dihalogen compounds in which both the halogen atoms are attached to the same carbon atom.
For example, Ethylidene dichloride
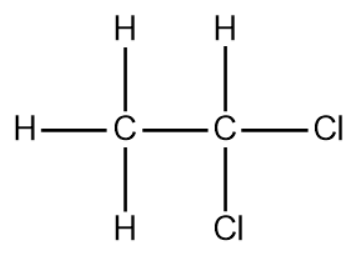
Suppose, there is a geminal dihalide CH3CH(Cl)2 is present, the hydrolysis of gem-halides takes place in alkaline medium. So, we will hydrolyse it in the presence of aqueous NaOH/KOH.
On hydrolysis, both of the chlorine group will be replaced by hydroxyl group (OH),
CH3CH(OH)2 will be formed.
As both the hydroxyl groups are present on the same carbon, this compound is not stable. So, water acts as a leaving group i.e. H2O will be removed.
Thus, forming an aldehyde group CH3CHO, this compound is called acetaldehyde. But this is the case when gem-dihalide is present at the terminal position.
CH3CH(Cl)2KOHaqCH3CH(OH)2→CH3COH+H2O
Now, let's take a compound CH3C(Cl)2CH3, on hydrolysis the chlorine groups will be replaced by hydroxyl groups, CH3C(OH)2CH3will be obtained.
Similarly, as the previous example, this compound will also be unstable, thus water will act as a leaving group.
Thus, forming a keto or carbonyl group CH3COCH3, this compound is called acetone.
CH3C(Cl)2CH3KOHaqCH3COCH3+2KCl+H2O
Therefore, from the above statements we can conclude that the correct option is (c).
Note : Geminal dihalides are prepared by reacting a non-enolizable aldehyde and/or ketone with phosgene or thionyl chloride in the presence of an organic-phosphorus compound.
