Question
Question: Gabriel phthalimide reaction is used for the preparation of ______ amines. (A) Primary aromatic ...
Gabriel phthalimide reaction is used for the preparation of ______ amines.
(A) Primary aromatic
(B) Secondary
(C) Primary aliphatic
(D) Tertiary
Solution
Hint: Knowing the attacking reagent in this case will help you identify the product. It should be noted that the relative strengths of reactants will determine which reagent will attack and which will be attacked.
Complete step by step solution:
The structure of phthalimide is as mentioned below:
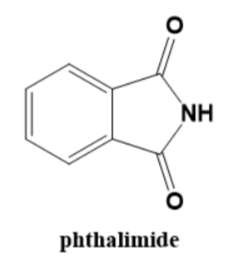
In the Gabriel phthalimide reaction phthalimide behaves as a nucleophile. The reaction is SN2or bimolecular nucleophilic substitution reaction.
The overall reaction can be divided into three steps, let us take it slow and go one by one:
- step 1
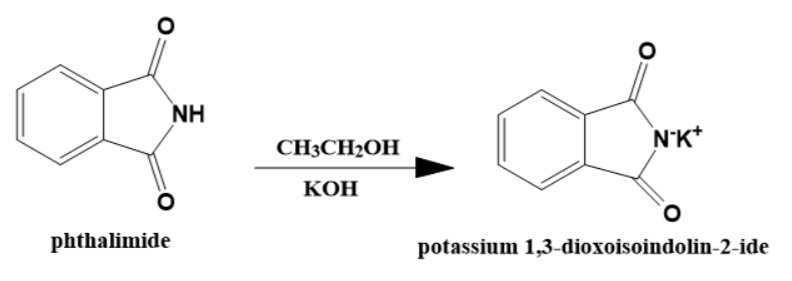
The phthalimide molecule reacts with ethanolic potassium hydroxide and converts into its potassium salt. This potassium salt scientifically named as potassium 1,3-dioxoisoindolin-2-ide is the nucleophile of this reaction.
- Step 2
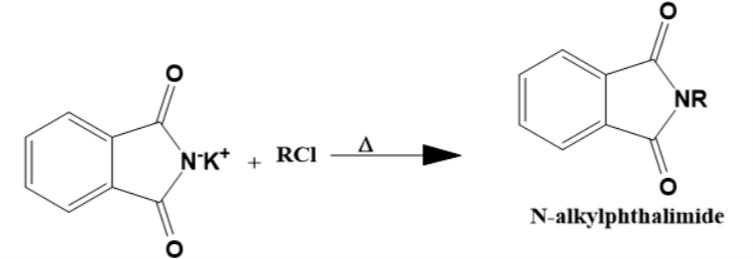
The potassium salt of phthalimide now reacts with an alkyl halide to form N-alkyl phthalimide when heated. This is the rate determining step in the overall reaction. As this step contains two reactants and a nucleophile is the active agent, this is SN2 in nature.
- Step 3
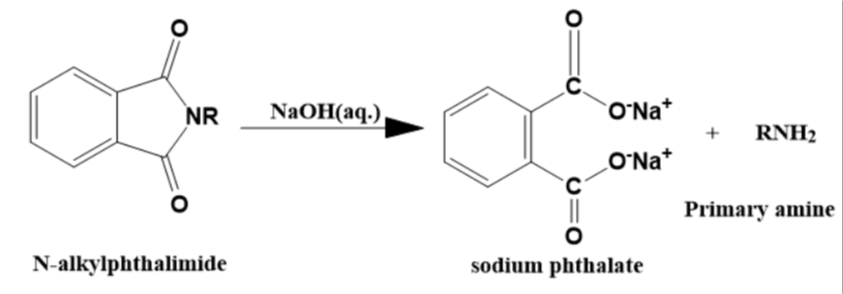
The N-alkyl phthalimide now reacts with the aqueous solution of sodium hydroxide and gives sodium phthalate and a primary amine as product.
As the nucleophile in this case is the potassium salt of phthalimide as mentioned in step-1 above, it can only react with a primary alkyl halide and not with any other degree because of its bulky structure and the resulting steric hindrance it will face. It also cannot attack an aromatic compound such as benzene because they usually do not undergo nucleophilic substitution reactions.
Gabriel phthalimide reaction therefore can only create primary aliphatic amines as mentioned in option (C).
Note:
Students mistake the alkyl halide to be the attacking reagent and therefore conclude that all degrees of aliphatic amines can be formed. Nucleophilicity depends less on the structure of a molecule and more on properties like:
- Which atom of the element is reactive? (In the above case it is the nitrogen atom on phthalimide)
- What is its electronegativity?
- And how reactive it is relative to the other compound.
These points should be asked to a given reaction to find its attacking reagent and then proceed accordingly.
