Question
Question: Fructose reduces Tollen’s reagent due to? A.Asymmetric carbons B.Primary alcoholic groups C.Se...
Fructose reduces Tollen’s reagent due to?
A.Asymmetric carbons
B.Primary alcoholic groups
C.Secondary alcoholic group
D.Enolization of fructose followed by conversion to aldehyde by base.
Solution
We must know about the word enolization. Enolization is a process in which aldehyde or ketone converts into enol or enolate form. The Keto group of fructose is undergoing enolization.
Complete step by step answer:
First we must know that the Tollen’s reagent is a chemical reagent. We can use Tollen’s reagent to analyze the presence of aldehyde and ketone functional groups. The chemical formula for Tollen’s reagent is [Ag(NH3)2]+. It can easily undergo decomposition. Therefore, we have to prepare freshly each time when we need.
We must know that aldehyde only reduces Tollen’s reagent. But in fructose, there's a ketone group.
Therefore, the ketone group of fructose is enolized in aqueous solution and then converted into aldehyde by base.
Now, the fructose also can reduce Tollen’s reagent.
Structure of fructose
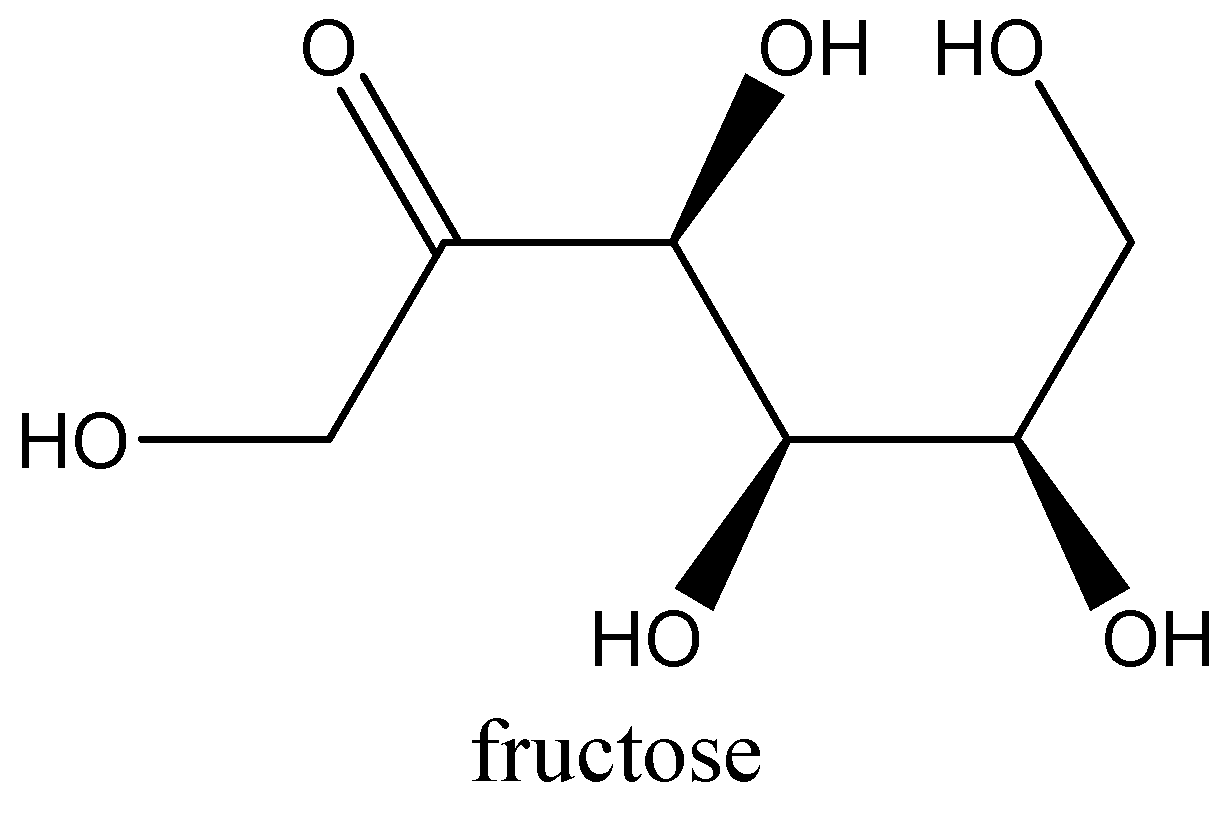
Additional information:
-Sucrose is also known as invert sugar, because on hydrolysis it will form invert sugars. Fructose is a polyhydroxy ketone molecule while glucose is polyhydroxy aldehyde.
-Fructose is known as ketose sugar, because it has a ketone functional group. Fructose exists in both forms of open chain as well as cyclic structure.
-Glucose and fructose both are hexose sugars. Glucose is an aldohexose monosaccharide while fructose is ketohexose monosaccharide. Glucose exists in both open chain as well as cyclic structure.
-Aldehydes also give positive Fehling tests.
Note:
We can also reduce α-hydroxy ketones by Tollen’s reagent due to enolization. Glucose and fructose are known as reducing sugars, because they can reduce the Tollen’s reagent. These are the simplest units of carbohydrates known as monosaccharides. Glucose and fructose are functional isomers. Fructose molecule has three chiral carbon atoms in its open chain structure. Glucose and fructose are combined to form disaccharide named as sucrose.
