Question
Question: From the phase diagram of water and an aqueous solution containing non volatile solute, identify the...
From the phase diagram of water and an aqueous solution containing non volatile solute, identify the correct options:
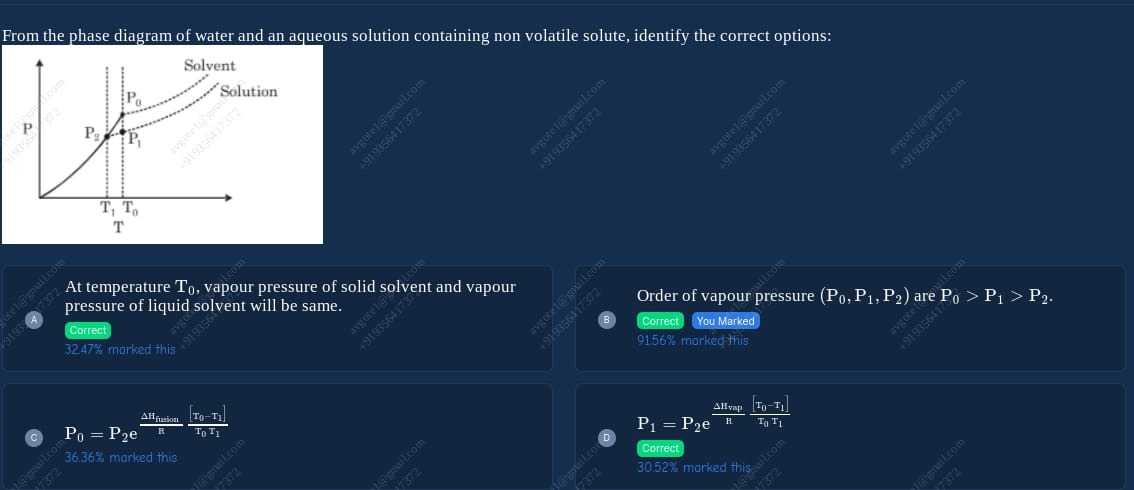
At temperature T0, vapour pressure of solid solvent and vapour pressure of liquid solvent will be same.
Order of vapour pressure (P0,P1,P2) are P0>P1>P2.
P0=P2eRΔHfusion[T0T1T0−T1]
P1=P2eRΔHvap[T0T1T0−T1]
All options A, B, C, and D are correct.
Solution
-
Option A: For a pure solvent, at its triple point (which occurs at T0), the vapor pressures of the solid and liquid phases are equal. Hence option A is correct.
-
Option B: In the diagram, at the same temperature T0 the vapor pressure of the solution (P1) is lowered (due to the presence of the nonvolatile solute) compared to that of the pure solvent (P0). Also, at a lower temperature T1 the vapor pressure on the solution curve is P2 (with P2<P1). Thus, the order is P0>P1>P2. Option B is correct.
-
Option C: The freezing point depression is derived using the Clausius–Clapeyron relation (applied to the fusion process). It leads to:
ln(P2P0)=RΔHfusion(T11−T01)Rewriting T11−T01=T0T1T0−T1 gives:
P0=P2exp[RΔHfusionT0T1T0−T1]So, option C is correct.
-
Option D: Similarly, applying the Clausius–Clapeyron equation to the vaporization process for the solution,
ln(P2P1)=RΔHvap(T11−T01)which rewrites to:
P1=P2exp[RΔHvapT0T1T0−T1]Making option D also correct.
