Question
Question: From the following reactions, identify the reaction that gives carboxylic acids as products. A. \(...
From the following reactions, identify the reaction that gives carboxylic acids as products.
A. CH3CH2CH2CH2OH1.KMnO4/KOH2.Dil.H2SO4
B. 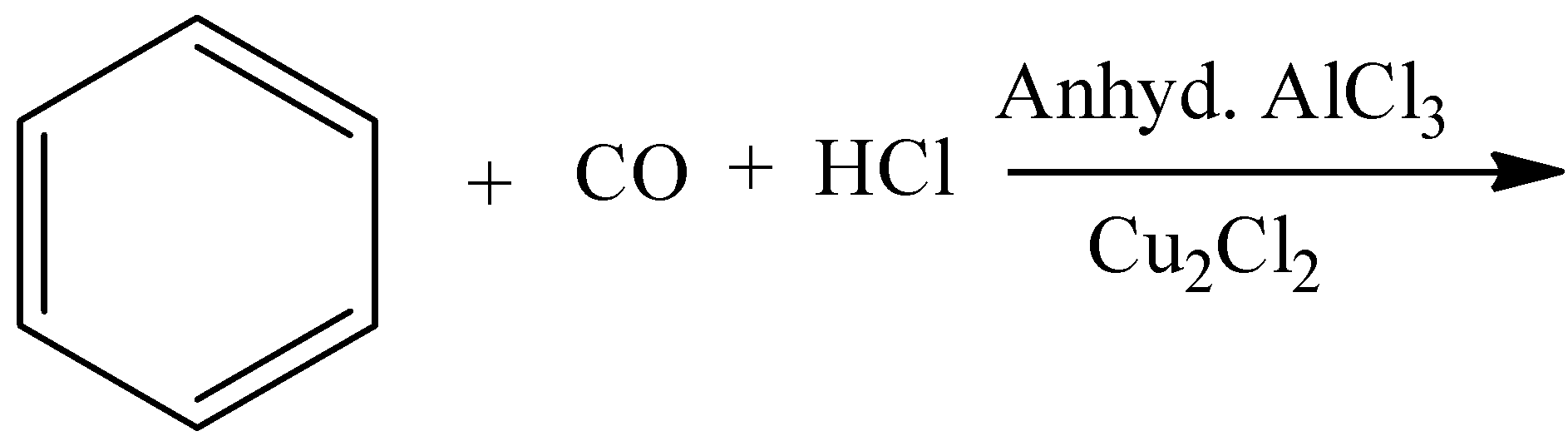
C. 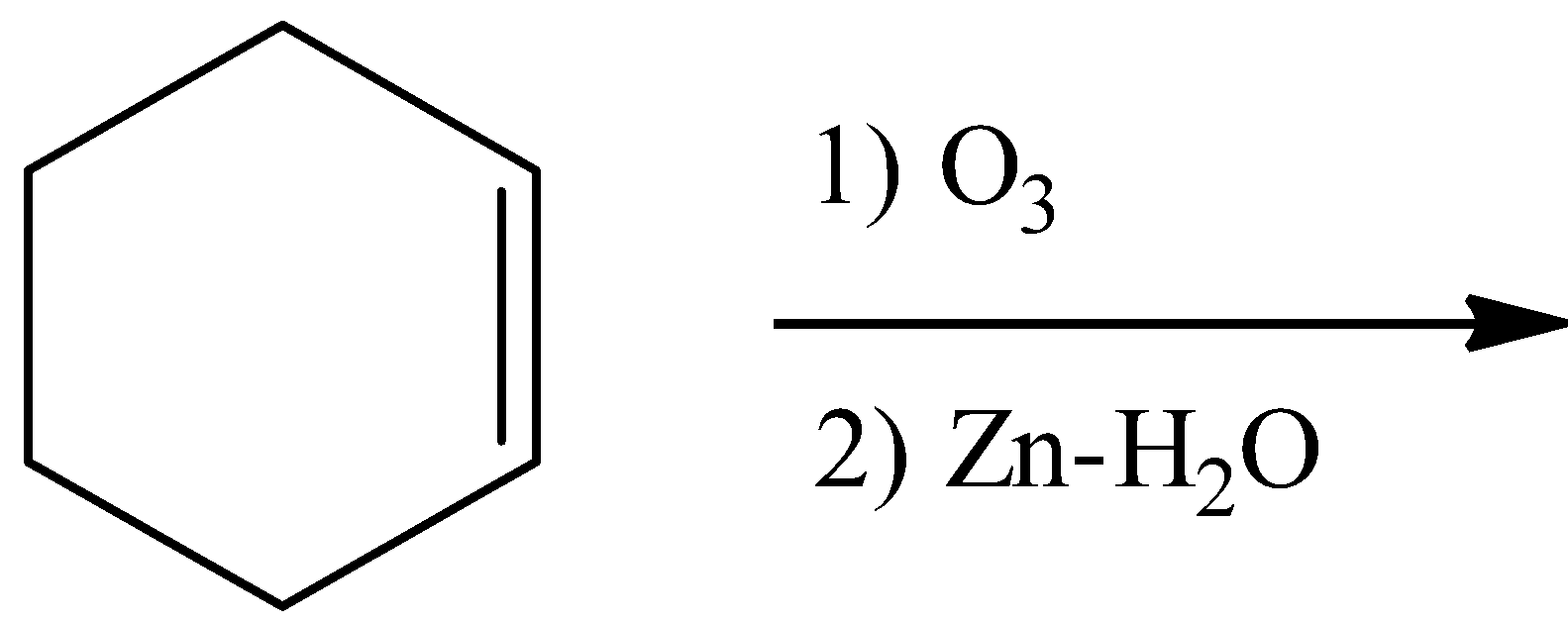
Solution
The functional group –COOH is called carboxylic acid functional group. Generally we need a strong oxidizing agent to prepare carboxylic acids from the reactants having different functional groups.
Complete step by step answer:
- In the question it is asked that among the given chemical reactions, identify the chemical reaction which will give carboxylic acid as the product.
- Coming to option A,
CH3CH2CH2CH2OH1.KMnO4/KOH2.Dil.H2SO4
- Generally primary alcohols react with strong oxidizing agents like potassium permanganate in the presence of an acid that produces carboxylic acid as the products.
CH3CH2CH2CH2OH1.KMnO4/KOH2.Dil.H2SO4CH3CH2CH2COOH
- Therefore butanol reacts with potassium permanganate in presence of dilute sulphuric acid and produces butanoic acid as the product.
- Coming to option B,
- In the above chemical reaction there is no strong oxidizing agent and there is no reactant which gives acid on oxidation.
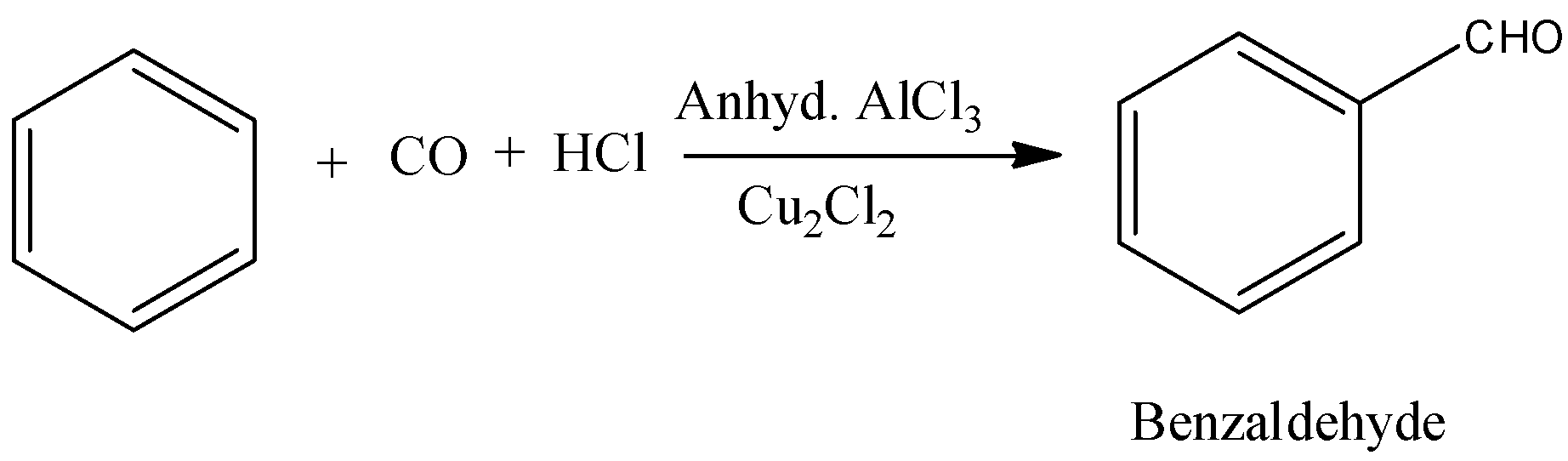
- The product formed in the above chemical reaction is an aldehyde.
- So, option B is wrong.
- Coming to option C,
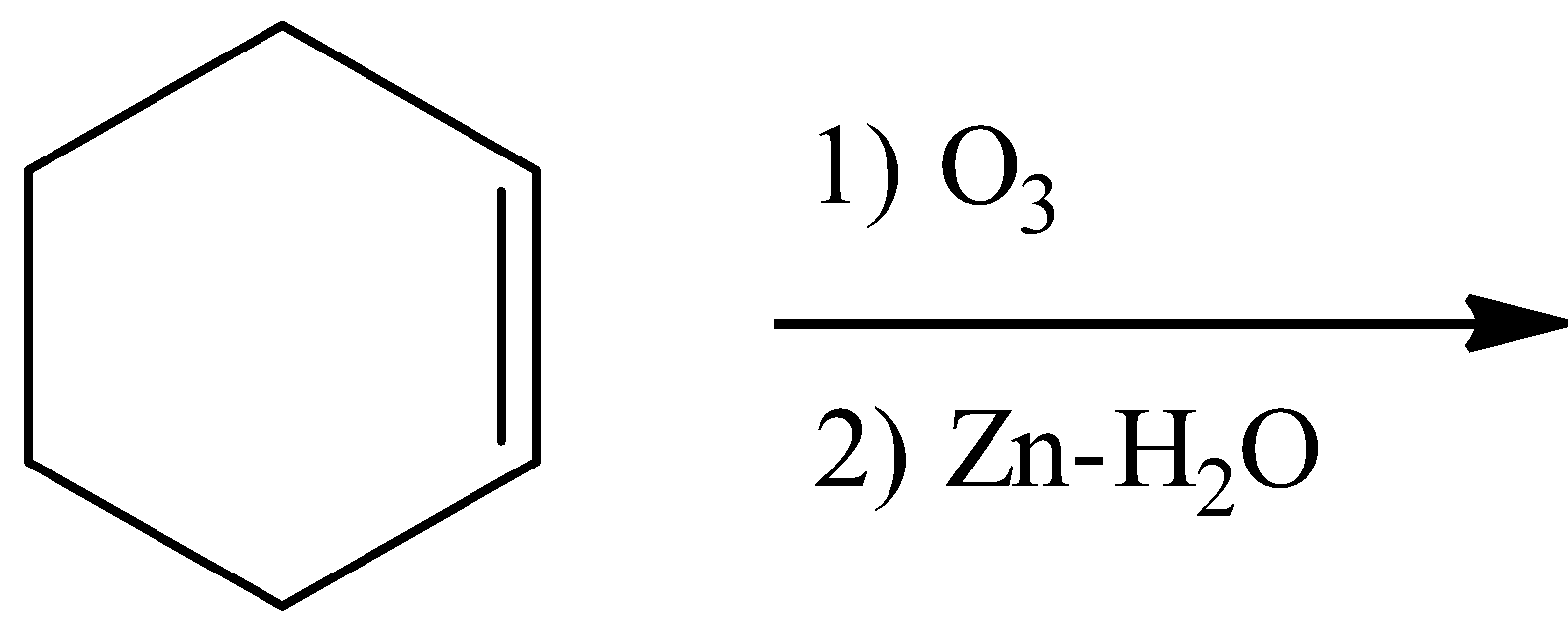
- Ozonolysis of the double bond produces either ketones or aldehydes as the products.
- So, the product in the chemical reaction in option C is as follows.
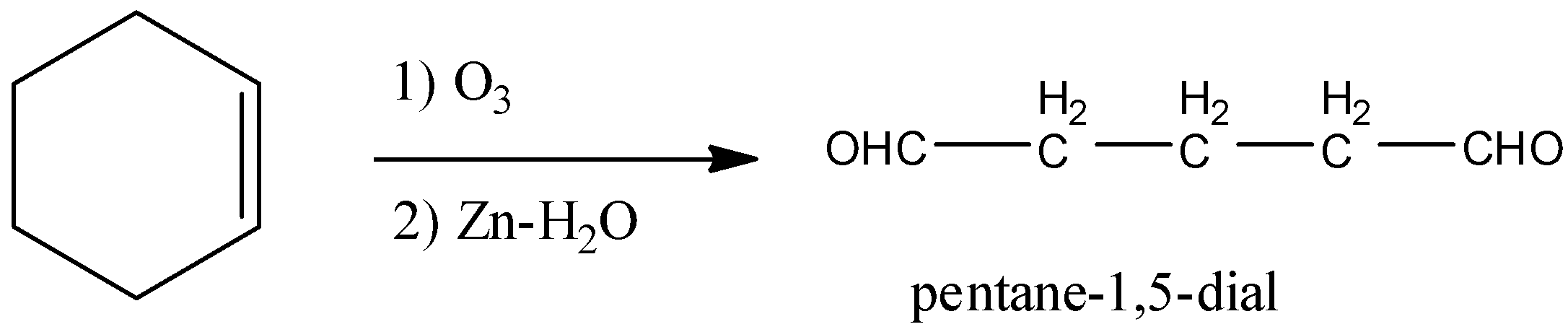
- So option C is also wrong.
- Therefore primary alcohols will give carboxylic acids on oxidation with strong oxidizing agents. The correct option is option “A” .
Note: The formation of benzaldehyde by using benzene, carbon monoxide and hydrochloric acid is called Gattermann Koch reaction. In this reaction aluminum trichloride is going to act as a catalyst. This reaction is an example of electrophilic substitution reaction.
